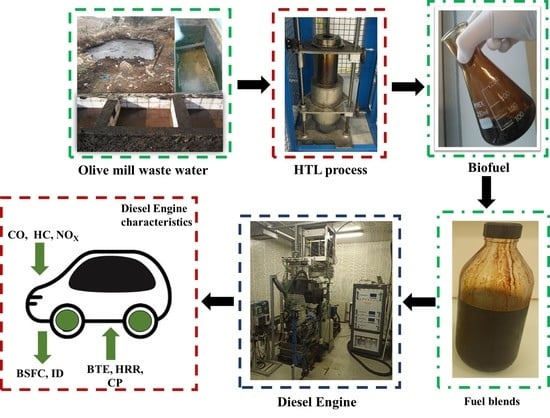
A Comprehensive Study on Effect of Biofuel Blending Obtained from Hydrothermal Liquefaction of Olive Mill Waste Water in Internal Combustion Engine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biofuel Production
2.2. Physicochemical Properties of Biofuel Blends
2.3. Experimental Engine Test
2.4. Model for Combustion Analysis
3. Results and Discussion
3.1. Combustion Characteristics
3.1.1. Cylinder Pressure
3.1.2. Heat Release Rate (HRR)
3.1.3. Ignition Delay
3.2. Engine Performance
3.3. Exhaust Emissions
3.3.1. HC and CO Emissions
3.3.2. NOx and Particulates Emissions
4. Conclusions
- Under full load conditions, the cylinder pressure curve of B10 closely resembled that of diesel, with a maximum value of 87 bars.
- The results showed that B10 leads to better performance compared to the other blends. This is due to several parameters such as better fuel atomization and low viscosity.
- At high loads, a reduction of 26% of CO emissions, and 11% of HC as well as PM emissions, were observed by using B10 blend in comparison with the B20 blend. In addition, a 10% improvement in brake thermal efficiency was noted.
- At high loads, B10 exhibits lower polluting effects than B30, with reductions of 43% in CO emissions, 10% in HC emissions, and 20% in particulate matter emissions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| P | Cylinder pressure, [bar] |
| V | Cylinder volume, [m3] |
| ᵞ | The ratio of specific heats, [-] |
| OMWW | Olive mill wastewater |
| Q | Heat, [J] |
| BTE | Brake thermal efficiency, [%] |
| HTL | Hydrothermal liquefaction |
| LHV | Lower heating value, [MJ/kg] |
| Mass flow rate, [kg/s] | |
| HHV | Higher heating value, [MJ/kg] |
| ID | Ignition delay, [deg CA] |
| Pb | Brake power, [kW] |
| L | Connecting rod length, [m] |
| TDC | Top dead center |
| BDC | Bottom dead center |
| Vd | Displacement volume, [m] |
| θ | Crank angle, [deg CA] |
| B0 | 100% diesel |
| CA | Crank angle |
| CI | Compression ignition |
| CO2 | Carbon dioxide |
| C | Stroke, [m] |
| PM | Particulate matter |
| HC | Hydrocarbon |
| B10 | Biofuel blends of 10% by volume of biofuel |
| B20 | Biofuel blends of 20% by volume of biofuel |
| B30 | Biofuel blends of 30% by volume of biofuel |
| CR | Compression ratio |
| NOx | Nitrogen oxides |
| CO | Carbon monoxide |
| Subscripts | |
| w | Wall of cylinder |
| c | Combustion |
| b | Brake |
References
- Hossain, F.M.; Kosinkova, J.; Brown, R.J.; Ristovski, Z.; Hankamer, B.; Stephens, E.; Rainey, T.J. Experimental Investigations of Physical and Chemical Large Batch Reactor. Energies 2017, 10, 467. [Google Scholar] [CrossRef] [Green Version]
- Hossain, F.M.; Nabi, N.; Rahman, M.; Bari, S.; Suara, K.; Ristovski, Z.; Brown, R.J. Experimental Investigation of Diesel Engine Performance, Combustion and Emissions Using a Novel Series of Dioctyl Phthalate (DOP) Biofuels Derived from Microalgae. Energies 2019, 12, 1964. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Reaction Parameters Effect on Hydrothermal Liquefaction of Castor (Ricinus Communis) Residue for Energy and Valuable Hydrocarbons Recovery. Renew. Energy 2019, 141, 1026–1041. [Google Scholar] [CrossRef]
- Coyle, W.T. The future of biofuels: A global perspective. J. Rural. Ment. Health 2007, 5, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Matayeva, A.; Bianchi, D.; Chiaberge, S.; Cavani, F.; Basile, F. Elucidation of reaction pathways of nitrogenous species by hydrothermal liquefaction process of model compounds. Fuel 2019, 240, 169–178. [Google Scholar] [CrossRef]
- Espadas-Aldana, G.; Vialle, C.; Belaud, J.P.; Vaca-Garcia, C.; Sablayrolles, C. Analysis and trends for Life Cycle Assessment of olive oil production. Sustain. Prod. Consum. 2019, 19, 216–230. [Google Scholar] [CrossRef] [Green Version]
- El Hassani, F.Z.; Fadile, A.; Faouzi, M.; Zinedine, A.; Merzouki, M.; Benlemlih, M. The long term effect of Olive Mill Wastewater (OMW) on organic matter humification in a semi-arid soil. Heliyon 2020, 6, e03181. [Google Scholar] [CrossRef] [Green Version]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Impact of Stability of Enriched Oil with Phenolic Extract from Olive MillWastewaters. Foods 2020, 9, 856. [Google Scholar] [CrossRef]
- Hadhoum, L.; Burnens, G.; Loubar, K.; Balistrou, M.; Tazerout, M. Bio-oil recovery from olive mill wastewater in sub-/supercritical alcohol- water system. Fuel 2019, 252, 360–370. [Google Scholar] [CrossRef]
- Kipçak, E.; Söǧüt, O.Ö.; Akgün, M. Hydrothermal gasification of olive mill wastewater as a biomass source in supercritical water. J. Supercrit. Fluids 2011, 57, 50–57. [Google Scholar] [CrossRef]
- Vitolo, S.; Petarca, L.; Bresci, B. Treatment of olive oil industry wastes. Bioresour. Technol. 1999, 67, 129–137. [Google Scholar] [CrossRef]
- Miranda, T.; Esteban, A.; Rojas, S.; Montero, I.; Ruiz, A. Combustion analysis of different olive residues. Int. J. Mol. Sci. 2008, 9, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Poerschmann, J.; Baskyr, I.; Weiner, B.; Koehler, R.; Wedwitschka, H.; Kopinke, F.D. Hydrothermal carbonization of olive mill wastewater. Bioresour. Technol. 2013, 133, 581–588. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Hadhoum, L.; Loubar, K.; Paraschiv, M.; Burnens, G.; Awad, S.; Tazerout, M. Optimization of oleaginous seeds liquefaction using response surface methodology. Biomass Convers. Biorefinery 2020, 11, 2655–2667. [Google Scholar] [CrossRef]
- Hadhoum, L.; Balistrou, M.; Burnens, G.; Loubar, K.; Tazerout, M. Hydrothermal liquefaction of oil mill wastewater for bio-oil production in subcritical conditions. Bioresour. Technol. 2016, 218, 9–17. [Google Scholar] [CrossRef]
- Haddad, K.; Jeguirim, M.; Jerbi, B.; Chouchene, A.; Dutournié, P.; Thevenin, N.; Ruidavets, L.; Jellali, S.; Limousy, L. Olive Mill Wastewater: From a Pollutant to Green Fuels, Agricultural Water Source and Biofertilizer. ACS Sustain. Chem. Eng. 2017, 5, 8988–8996. [Google Scholar] [CrossRef]
- Elleuch, A.; Halouani, K.; Li, Y. Investigation of direct-fed Solid Oxide Fuel Cell fueled by upgraded bio-oil extracted from olive waste pyrolysis: Part 1: Bio-oil characterization and preliminary cell testing. Energy Technol. 2018, 300072, 53–60. [Google Scholar] [CrossRef]
- Wu, X.; Daniel, R.; Tian, G.; Xu, H.; Huang, Z.; Richardson, D. Dual-injection: The flexible, bi-fuel concept for spark-ignition engines fuelled with various gasoline and biofuel blends. Appl. Energy 2011, 88, 2305–2314. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Rakopoulos, C.D.; Dimaratos, A.M.; Rakopoulos, D.C. Exhaust emissions of diesel engines operating under transient conditions with biodiesel fuel blends. Prog. Energy Combust. Sci. 2012, 38, 691–715. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Rakopoulos, D.C.; Rakopoulos, C.D. Combustion noise radiation during dynamic diesel engine operation including effects of various biofuel blends: A review. Renew. Sustain. Energy Rev. 2016, 54, 1099–1113. [Google Scholar] [CrossRef]
- Abed, K.A.; Gad, M.S.; El Morsi, A.K.; Sayed, M.M.; Elyazeed, S.A. Effect of biodiesel fuels on diesel engine emissions. Egypt. J. Pet. 2019, 28, 183–188. [Google Scholar] [CrossRef]
- Komninos, N.P.; Rakopoulos, C.D. Modeling HCCI combustion of biofuels: A review. Renew. Sustain. Energy Rev. 2012, 16, 1588–1610. [Google Scholar] [CrossRef]
- Giakoumis, E.G. A statistical investigation of biodiesel effects on regulated exhaust emissions during transient cycles. Appl. Energy 2012, 98, 273–291. [Google Scholar] [CrossRef]
- Debnath, B.K.; Sahoo, N.; Saha, U.K. Adjusting the operating characteristics to improve the performance of an emulsified palm oil methyl ester run diesel engine. Energy Convers. Manag. 2013, 69, 191–198. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Periyasamy, M.; Prathima, A.; Sabariswaran, K. Performance analysis of diesel engine fueled with S. marginatum Macro algae biofuel—Diesel blends. Mater. Today Proc. 2020, 33, 3464–3469. [Google Scholar] [CrossRef]
- Kumar, P.S.; Donga, R.K.; Sahoo, P.K. Experimental comparative study between performance and emissions of jatropha biodiesel and diesel under varying injection pressures. Int. J. Eng. Sci. Emerg. Technol. 2012, 3, 98–112. [Google Scholar]
- Kuti, O.A.; Zhu, J.; Nishida, K.; Wang, X.; Huang, Z. Characterization of spray and combustion processes of biodiesel fuel injected by diesel engine common rail system. Fuel 2013, 104, 838–846. [Google Scholar] [CrossRef]
- Nabi, M.N.; Rahman, M.M.; Islam, M.A.; Hossain, F.M.; Brooks, P.; Rowlands, W.N.; Tulloch, J.; Ristovski, Z.D.; Brown, R.J. Fuel characterisation, engine performance, combustion and exhaust emissions with a new renewable Licella biofuel. Energy Convers. Manag. 2015, 96, 588–598. [Google Scholar] [CrossRef]
- Wong, P.K.; Ghadikolaei, M.A.; Chen, S.H.; Fadairo, A.A.; Ng, K.W.; Lee, S.M.Y.; Xu, J.C.; Lian, Z.D.; Li, S.; Wong, H.C.; et al. Physicochemical and cell toxicity properties of particulate matter (PM) from a diesel vehicle fueled with diesel, spent coffee ground biodiesel, and ethanol. Sci. Total Environ. 2022, 824, 153873. [Google Scholar] [CrossRef]
- Altarazi, Y.S.M.; Abu Talib, A.R.; Yu, J.; Gires, E.; Abdul Ghafir, M.F.; Lucas, J.; Yusaf, T. Effects of biofuel on engines performance and emission characteristics: A review. Energy 2022, 238, 121910. [Google Scholar] [CrossRef]
- Imdadul, H.K.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Alabdulkarem, A.; Rashed, M.M.; Teoh, Y.H.; How, H.G. Higher alcohol-biodiesel-diesel blends: An approach for improving the performance, emission, and combustion of a light-duty diesel engine. Energy Convers. Manag. 2016, 111, 174–185. [Google Scholar] [CrossRef]
- Imdadul, H.K.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Alabdulkarem, A.; Rashed, M.M.; Ashraful, A.M. Influences of ignition improver additive on ternary (diesel-biodiesel-higher alcohol) blends thermal stability and diesel engine performance. Energy Convers. Manag. 2016, 123, 252–264. [Google Scholar] [CrossRef]
- Aklouche, F.Z.; Loubar, K.; Bentebbiche, A.; Awad, S.; Tazerout, M. Experimental investigation of the equivalence ratio influence on combustion, performance and exhaust emissions of a dual fuel diesel engine operating on synthetic biogas fuel. Energy Convers. Manag. 2017, 152, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Aklouche, F.Z.; Loubar, K.; Bentebbiche, A.; Awad, S.; Tazerout, M. Predictive model of the diesel engine operating in dual-fuel mode fuelled with different gaseous fuels. Fuel 2018, 220, 599–606. [Google Scholar] [CrossRef]
- Bora, B.J.; Saha, U.K.; Chatterjee, S.; Veer, V. Effect of compression ratio on performance, combustion and emission characteristics of a dual fuel diesel engine run on raw biogas. Energy Convers. Manag. 2014, 87, 1000–1009. [Google Scholar] [CrossRef]
- Fayette Taylor, C. Internal Combustion Engine in Theory and Practice: Combustion, Fuels, Materials, Design; Revised; MIT Press: Cambridge, MA, USA, 1985; Volume 2. [Google Scholar] [CrossRef]
- Gumus, M. A comprehensive experimental investigation of combustion and heat release characteristics of a biodiesel (hazelnut kernel oil methyl ester) fueled direct injection compression ignition engine. Fuel 2010, 89, 2802–2814. [Google Scholar] [CrossRef]
- Niculescu, R.; Clenci, A.; Iorga-Siman, V. Review on the use of diesel-biodiesel-alcohol blends in compression ignition engines. Energies 2019, 12, 1194. [Google Scholar] [CrossRef] [Green Version]
- Nour, M.; Attia, A.M.A.; Nada, S.A. Combustion, performance and emission analysis of diesel engine fuelled by higher alcohols (butanol, octanol and heptanol)/diesel blends. Energy Convers. Manag. 2019, 185, 313–329. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; Tata McGraw-Hill Education Private Limited: New Delhi, India, 2013. [Google Scholar]
- Łagowski, P.; Wcisło, G.; Kurczyński, D. Comparison of the Combustion Process Parameters in a Diesel Engine Powered by Second-Generation Biodiesel Compared to the First-Generation Biodiesel. Energies 2022, 15, 6835. [Google Scholar] [CrossRef]
- EL_Kassaby, M.; Nemit_allah, M.A. Studying the effect of compression ratio on an engine fueled with waste oil produced biodiesel/diesel fuel. Alex. Eng. J. 2013, 52, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Panwar, N.L.; Shrirame, H.Y.; Rathore, N.S.; Jindal, S.; Kurchania, A.K. Performance evaluation of a diesel engine fueled with methyl ester of castor seed oil. Appl. Therm. Eng. 2010, 30, 245–249. [Google Scholar] [CrossRef]
- Tanwar, M.D.; Torres, F.A.; Alqahtani, A.M.; Tanwar, P.K.; Bhand, Y.; Doustdar, O. Promising Bioalcohols for Low-Emission Vehicles. Energies 2023, 16, 597. [Google Scholar] [CrossRef]
- Di, Y.; Cheung, C.S.; Huang, Z. Experimental investigation on regulated and unregulated emissions of a diesel engine fueled with ultra-low sulfur diesel fuel blended with biodiesel from waste cooking oil. Sci. Total Environ. 2009, 407, 835–846. [Google Scholar] [CrossRef]
- Cheung, C.S.; Zhu, L.; Huang, Z. Regulated and unregulated emissions from a diesel engine fueled with biodiesel and biodiesel blended with methanol. Atmos. Environ. 2009, 43, 4865–4872. [Google Scholar] [CrossRef]
- Muralidharan, K.; Vasudevan, D. Performance, emission and combustion characteristics of a variable compression ratio engine using methyl esters of waste cooking oil and diesel blends. Appl. Energy 2011, 88, 3959–3968. [Google Scholar] [CrossRef]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Emberson, D.R.; Wyndorps, J.; Ahmed, A.; Pires Bjørgen, K.O.; Løvås, T. Detailed examination of the combustion of diesel and glycerol emulsions in a compression ignition engine. Fuel 2021, 291, 120147. [Google Scholar] [CrossRef]
- ÇAKMAK, A.; ÖZCAN, H. Evaluation of glycerol tert-butyl ethers as renewable fuel additive. Int. J. Automot. Eng. Technol. 2020, 9, 66–75. [Google Scholar] [CrossRef]
- De, B.; Panua, R.S. An experimental study on performance and emission characteristics of vegetable oil blends with diesel in a direct injection variable compression ignition engine. Procedia Eng. 2014, 90, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Balaji, G.; Cheralathan, M. Experimental investigation of antioxidant effect on oxidation stability and emissions in a methyl ester of neem oil fueled DI diesel engine. Renew. Energy 2015, 74, 910–916. [Google Scholar] [CrossRef]
- Alagu, R.M.; Sundaram, E.G. Preparation and characterization of pyrolytic oil through pyrolysis of neem seed and study of performance, combustion and emission characteristics in CI engine. J. Energy Inst. 2018, 91, 100–109. [Google Scholar] [CrossRef]









| Proximate Analysis (wt.%) | Ultimate Analysis (On Dry Basis, wt.%) | Chemical Composition (On Dry Basis, wt.%) | |||
|---|---|---|---|---|---|
| Moisture, as received | 78.50 | C | 46.87 | Hemicellulose | 36.90 |
| Ash (on a dry basis) | 21.87 | H | 6.88 | Cellulose | 19.47 |
| Volatiles | 58.70 | N | 2.40 | Lignin | 28.55 |
| Fixed carbon | 24.26 | S | n.d b | Fat, as received | 14.30 |
| O a | 21.98 | HHV (MJ/kg) | 24.26 | ||
| Bore × stroke | 95.3 mm × 88.9 mm |
| Cooling system | Air-cooled |
| Compression ratio | 18:1 |
| General details | 4-Stroke, single cylinder, naturally aspirated, |
| Injection system | Direct injection |
| Orifices × diameter | 4 × 0.25 mm |
| Connecting rod length | 165.3 mm |
| Piston type | Cylindrical bowl (diameter: 45 mm and depth: 15 mm) |
| Displacement volume | 630 cm3 |
| injection timing | 13° BTDC |
| injection pressure | 240 bar |
| Rated power output | 4.5 kW at 1500 rpm |
| EVO | 76°CA BBDC |
| IVC | 69°CA ABDC |
| IVO | 36°CA BTDC |
| EVC | 32°CA ATDC |
| Measurements | Sensor Type | Accuracy | |
|---|---|---|---|
| Torque | Effort sensor (FN 3148) | ±0.1 N·m | |
| Speed | AVL 365C | ±3 rpm | |
| Injection timing | AVL 365C | ±0.05 °CA | |
| Intake air flow rate | Differential pressure transmitter (LPX5841) | ±1.0% | |
| Temperature of exhaust gas | K type | ±1.6 °C | |
| Temperature of ambient air | HD 2012 TC/150 | ±0.2 °C | |
| Temperature of injected fuel | K type | ±1.6 °C | |
| Cylinder pressure | Piezo-electric (AVL QH32D) | ±2 bars | |
| Injection pressure | Piezo-electric (AVL QH33D) | ±2 bars | |
| Fuel mass flow rate | Coriolis type (RHM015)(RHM015) | ±0.5% | |
| NOx | chemiluminescence (TOPAZE 32M) | ±100 ppm | |
| HC | FID (Graphite 52M) | ±10 ppm | |
| CO, CO2, O2 | Infra-red detector (MIR 2M) | ±50 ppm | |
| Particulates | Electric (Pegasor Particle Sensor) | ±1 μg/m3 | |
| Calculated results | Uncertainty range (%) | ||
| Air/Fuel equivalence ratio | 1.1 | ||
| BSFC | 0.6–2.0 | ||
| BTE | 0.7–2.0 | ||
| Brake power | 0.4–1.9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aklouche, F.Z.; Hadhoum, L.; Loubar, K.; Tazerout, M. A Comprehensive Study on Effect of Biofuel Blending Obtained from Hydrothermal Liquefaction of Olive Mill Waste Water in Internal Combustion Engine. Energies 2023, 16, 2534. https://doi.org/10.3390/en16062534
Aklouche FZ, Hadhoum L, Loubar K, Tazerout M. A Comprehensive Study on Effect of Biofuel Blending Obtained from Hydrothermal Liquefaction of Olive Mill Waste Water in Internal Combustion Engine. Energies. 2023; 16(6):2534. https://doi.org/10.3390/en16062534
Chicago/Turabian StyleAklouche, Fatma Zohra, Loubna Hadhoum, Khaled Loubar, and Mohand Tazerout. 2023. "A Comprehensive Study on Effect of Biofuel Blending Obtained from Hydrothermal Liquefaction of Olive Mill Waste Water in Internal Combustion Engine" Energies 16, no. 6: 2534. https://doi.org/10.3390/en16062534
APA StyleAklouche, F. Z., Hadhoum, L., Loubar, K., & Tazerout, M. (2023). A Comprehensive Study on Effect of Biofuel Blending Obtained from Hydrothermal Liquefaction of Olive Mill Waste Water in Internal Combustion Engine. Energies, 16(6), 2534. https://doi.org/10.3390/en16062534