Review of Concentrated Solar Power Technology Applications in Photocatalytic Water Purification and Energy Conversion: Overview, Challenges and Future Directions
Abstract
:1. Introduction
2. Feasibility of Solar Energy for Photocatalytic Applications
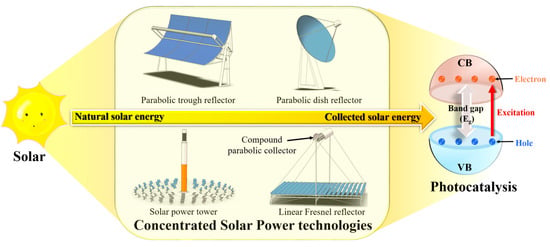
3. Brief Overview of Concentrated Solar Power Technologies
3.1. Parabolic Trough Reflectors
3.2. Parabolic Dish Reflectors
3.3. Solar Power Towers
3.4. Linear Fresnel Reflectors
4. CSP Technologies Applied in Photocatalysis
4.1. Solar Concentrated Type
4.1.1. Parabolic Trough Reflector-Based Photoreactors
4.1.2. Parabolic Dish Reflector-Based Photoreactors
4.1.3. Fresnel Condenser-Based Photoreactors
4.1.4. Problems in Solar-Concentrated Photoreactors
4.2. Non/Low-Concentrated Photoreactors
4.2.1. V-Groove-Based Photoreactors
4.2.2. Compound Parabolic Collector-Based Photoreactors
5. Relevant Problems in CSP-Based Photoreactors
5.1. Instability of Real Weather
5.2. Nighttime Operation
5.3. Configuration of Photocatalyst Immobilization Substrate
5.4. Economic Analysis
6. Prospects for Further Work
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, C.; Ming, J.; Sun, X.; Zhu, Y.; An, G.; Chen, G.; Yang, Y. Development of a Green and Efficient Photocatalytic Mesh Microalgae Biorefinery (PMMB) System for Sustainable Biomass Conversion under Real Solar Light. Chem. Eng. J. 2023, 466, 143260. [Google Scholar] [CrossRef]
- Akpan, J.O.O. Sustainable Energy Development: History and Recent Advances. Energies 2023, 16, 7049. [Google Scholar] [CrossRef]
- Ming, J.; Sun, X.; Ma, Q.; Liu, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Yang, Y. Advanced Photocatalytic Sterilization for Recalcitrant Enterococcus Sp. Contaminated Water by Newly Developed Z-Scheme Bi2WO6 Based Composites under Solar Light. Chemosphere 2023, 310, 136912. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Hu, X.; Liu, N.; Sharma, A.; Zhang, C.; Kawazoe, N.; Chen, G.; Yang, Y. Polyethylene Glycol (PEG)-Modified Ag/Ag2O/Ag3PO4/Bi2WO6 Photocatalyst Film with Enhanced Efficiency and Stability under Solar Light. J. Colloid Interface Sci. 2020, 569, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liu, N.; Ma, Q.; Sharma, A.; Nagai, D.; Sun, X.; Zhang, C.; Yang, Y. Sol-Gel/Hydrothermal Two-Step Synthesis Strategy for Promoting Ag Species–Modified TiO2-Based Composite Activity toward H2 Evolution under Solar Light. Mater. Today Energy 2021, 20, 100648. [Google Scholar] [CrossRef]
- Hu, X.; Ma, Q.; Wang, X.; Yang, Y.; Liu, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Yang, Y. Layered Ag/Ag2O/BiPO4/Bi2WO6 Heterostructures by Two-Step Method for Enhanced Photocatalysis. J. Catal. 2020, 387, 28–38. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, N.; Ming, J.; Sharma, A.; Ma, Q.; Liu, Z.; Chen, G.; Yang, Y. Development of a Novel Solar Energy Controllable Linear Fresnel Photoreactor (LFP) for High-Efficiency Photocatalytic Wastewater Treatment under Actual Weather. Water Res. 2022, 208, 117880. [Google Scholar] [CrossRef]
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet Effect of Single-Crystalline Ag3PO4 Sub-Microcrystals on Photocatalytic Properties. J. Am. Chem. Soc. 2011, 4, 6490–6492. [Google Scholar] [CrossRef]
- Portela, R.; Suárez, S.; Tessinari, R.F.; Hernández-Alonso, M.D.; Canela, M.C.; Sánchez, B. Solar/Lamp-Irradiated Tubular Photoreactor for Air Treatment with Transparent Supported Photocatalysts. Appl. Catal. B Environ. 2011, 105, 95–102. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, Q.; Zhang, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Negishi, N.; Yang, Y. Superior Disinfection Effect of Escherichia Coli by Hydrothermal Synthesized TiO2-Based Composite Photocatalyst under LED Irradiation: Influence of Environmental Factors and Disinfection Mechanism. Environ. Pollut. 2019, 247, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lan, Z.A.; Lin, L.; Lin, S.; Wang, X. Overall Water Splitting by Pt/g-C3N4 Photocatalysts without Using Sacrificial Agents. Chem. Sci. 2016, 7, 3062–3066. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z. Metal-Free Efficient Photocatalyst for Stable Visible Water Splitting via a Two-Electron Pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef]
- Reli, M.; Edelmannová, M.; Šihor, M.; Praus, P.; Svoboda, L.; Mamulová, K.K.; Otoupalíková, H.; Čapek, L.; Hospodková, A.; Obalová, L.; et al. Photocatalytic H2 Generation from Aqueous Ammonia Solution Using ZnO Photocatalysts Prepared by Different Methods. Int. J. Hydrogen Energy 2015, 40, 8530–8538. [Google Scholar] [CrossRef]
- Kominami, H.; Nishimune, H.; Ohta, Y.; Arakawa, Y.; Inaba, T. Photocatalytic Hydrogen Formation from Ammonia and Methyl Amine in an Aqueous Suspension of Metal-Loaded Titanium(IV) Oxide Particles. Appl. Catal. B Environ. 2012, 111–112, 297–302. [Google Scholar] [CrossRef]
- Vidyasagar, D.; Ghugal, S.G.; Kulkarni, A.; Mishra, P.; Shende, A.G.; Jagannath; Umare, S.S.; Sasikala, R. Silver/Silver(II) Oxide (Ag/AgO) Loaded Graphitic Carbon Nitride Microspheres: An Effective Visible Light Active Photocatalyst for Degradation of Acidic Dyes and Bacterial Inactivation. Appl. Catal. B Environ. 2018, 221, 339–348. [Google Scholar] [CrossRef]
- Deng, J.; Liang, J.; Li, M.; Tong, M. Enhanced Visible-Light-Driven Photocatalytic Bacteria Disinfection by g-C3N4-AgBr. Colloids Surf. B Biointerfaces 2017, 152, 49–57. [Google Scholar] [CrossRef]
- Braham, R.J.; Harris, A.T. Review of Major Design and Scale-up Considerations for Solar Photocatalytic Reactors. Ind. Eng. Chem. Res. 2009, 48, 8890–8905. [Google Scholar] [CrossRef]
- Ajbar, W.; Hernández, J.A.; Parrales, A.; Torres, L. Thermal Efficiency Improvement of Parabolic Trough Solar Collector Using Different Kinds of Hybrid Nanofluids. Case Stud. Therm. Eng. 2023, 42, 102759. [Google Scholar] [CrossRef]
- Desai, N.B.; Bandyopadhyay, S. Optimization of Concentrating Solar Thermal Power Plant Based on Parabolic Trough Collector. J. Clean. Prod. 2015, 89, 262–271. [Google Scholar] [CrossRef]
- Ma, Q.; Ming, J.; Sun, X.; Liu, N.; Chen, G.; Yang, Y. Visible Light Active Graphene Oxide Modified Ag/Ag2O/BiPO4/Bi2WO6 for Photocatalytic Removal of Organic Pollutants and Bacteria in Wastewater. Chemosphere 2022, 306, 135512. [Google Scholar] [CrossRef]
- Jafarova, V.N.; Orudzhev, G.S. Structural and Electronic Properties of ZnO: A First-Principles Density-Functional Theory Study within LDA(GGA) and LDA(GGA)+U Methods. Solid State Commun. 2021, 325, 114166. [Google Scholar] [CrossRef]
- Huang, C.K.; Wu, T.; Huang, C.W.; Lai, C.Y.; Wu, M.Y.; Lin, Y.W. Enhanced Photocatalytic Performance of BiVO4 in Aqueous AgNO3 Solution under Visible Light Irradiation. Appl. Surf. Sci. 2017, 399, 10–19. [Google Scholar] [CrossRef]
- Lee, S.S.; Bai, H.; Liu, Z.; Sun, D.D. Electrospun TiO2/SnO2 Nanofibers with Innovative Structure and Chemical Properties for Highly Efficient Photocatalytic H2 Generation. Int. J. Hydrogen Energy 2012, 37, 10575–10584. [Google Scholar] [CrossRef]
- Yao, W.; Song, X.; Huang, C.; Xu, Q.; Wu, Q. Enhancing Solar Hydrogen Production via Modified Photochemical Treatment of Pt/CdS Photocatalyst. Catal. Today 2013, 199, 42–47. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, Q.; Zhang, X.; Peng, T. Graphite Oxide-TiO2 Nanocomposite and Its Efficient Visible-Light-Driven Photocatalytic Hydrogen Production. J. Alloys Compd. 2012, 516, 85–90. [Google Scholar] [CrossRef]
- Kim, J.; Kang, M. High Photocatalytic Hydrogen Production over the Band Gap-Tuned Urchin-like Bi2S3-Loaded TiO2 Composites System. Int. J. Hydrogen Energy 2012, 37, 8249–8256. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, B.; Yu, J. A New Understanding of the Photocatalytic Mechanism of the Direct Z-Scheme g-C3N4/TiO2 Heterostructure. Phys. Chem. Chem. Phys. 2016, 18, 31175–31183. [Google Scholar] [CrossRef]
- Wunderlich, W.; Oekermann, T.; Miao, L.; Hue, N.T.; Tanemura, S.; Tanemura, M. Electronic Properties of Nano-Porous TiO2- and ZnO-Thin Films-Comparison of Simulations and Experiments. J. Ceram. Process. Res. 2004, 5, 343–354. [Google Scholar]
- Lei, Z.; You, W.; Liu, M.; Zhou, G.; Takata, T.; Hara, M.; Domen, K.; Li, C. Photocatalytic Water Reduction under Visible Light on a Novel ZnIn2S4 Catalyst Synthesized by Hydrothermal Method. Chem. Commun. 2003, 3, 2142–2143. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yu, H.; Yu, J.; Liu, S. Ag2O as a New Visible-Light Photocatalyst: Self-Stability and High Photocatalytic Activity. Chem. A Eur. J. 2011, 17, 7777–7780. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, Q.; Wang, X.; Kawazoe, N.; Yang, Y. Nonmetal-Metal-Semiconductor-Promoted P/Ag/Ag2O/Ag3PO4/TiO2 Photocatalyst with Superior Photocatalytic Activity and Stability. J. Mater. Chem. A 2015, 3, 17858–17865. [Google Scholar] [CrossRef]
- Maeda, K.; Lu, D.; Domen, K. Direct Water Splitting into Hydrogen and Oxygen under Visible Light by Using Modified Taon Photocatalysts with D0 Electronic Configuration. Chem. A Eur. J. 2013, 19, 4986–4991. [Google Scholar] [CrossRef]
- Kim, H.G.; Hwang, D.W.; Kim, J.; Kim, Y.G.; Lee, J.S. Highly Donor-Doped (110) Layered Perovskite Materials as Novel Photocatalysts for Overall Water Splitting. Chem. Commun. 1999, 2, 1077–1078. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Gupta, N.M. Factors Affecting the Production of H2 by Water Splitting over a Novel Visible-Light-Driven Photocatalyst GaFeO3. Int. J. Hydrogen Energy 2012, 37, 4897–4907. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, H.X.; Chen, Y.P.; Meng, X.Y.; Ghanbaja, J.; Horwat, D.; Pierson, J.F. Wurtzite CoO: A Direct Band Gap Oxide Suitable for a Photovoltaic Absorber. Chem. Commun. 2018, 54, 13949–13952. [Google Scholar] [CrossRef]
- Hu, S.P.; Xu, C.Y.; Zhen, L. Solvothermal Synthesis of Bi2WO6 Hollow Structures with Excellent Visible-Light Photocatalytic Properties. Mater. Lett. 2013, 95, 117–120. [Google Scholar] [CrossRef]
- Basu, M.; Sinha, A.K.; Pradhan, M.; Sarkar, S.; Negishi, Y.; Govind; Pal, T. Evolution of Hierarchical Hexagonal Stacked Plates of CuS from Liquid—Liquid Interface and Its Photocatalytic Application for Oxidative Degradation of Different Dyes under Indoor Lighting. Environ. Sci. Technol. 2010, 44, 6313–6318. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Flores, P.; Poon, P.S.; Ania, C.O.; Matos, J. Performance of a C-Containing Cu-Based Photocatalyst for the Degradation of Tartrazine: Comparison of Performance in a Slurry and CPC Photoreactor under Artificial and Natural Solar Light. J. Colloid Interface Sci. 2022, 623, 646–659. [Google Scholar] [CrossRef]
- Reutergårdh, L.B.; Iangphasuk, M. Photocatalytic Decolourization of Reactive Azo Dye: A Comparison between TiO2 and CdS Photocatalysis. Chemosphere 1997, 35, 585–596. [Google Scholar] [CrossRef]
- Askari, N.; Beheshti, M.; Mowla, D.; Farhadian, M. Facile Construction of Novel Z-Scheme MnWO4/Bi2S3 Heterojunction with Enhanced Photocatalytic Degradation of Antibiotics. Mater. Sci. Semicond. Process. 2021, 127, 105723. [Google Scholar] [CrossRef]
- Yu, J.; Kiwi, J.; Zivkovic, I.; Rønnow, H.M.; Wang, T.; Rtimi, S. Quantification of the Local Magnetized Nanotube Domains Accelerating the Photocatalytic Removal of the Emerging Pollutant Tetracycline. Appl. Catal. B Environ. 2019, 248, 450–458. [Google Scholar] [CrossRef]
- Castro-Alférez, M.; Polo-López, M.I.; Marugán, J.; Fernández-Ibáñez, P. Mechanistic Model of the Escherichia Coli Inactivation by Solar Disinfection Based on the Photo-Generation of Internal ROS and the Photo-Inactivation of Enzymes: CAT and SOD. Chem. Eng. J. 2017, 318, 214–223. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of Photocatalytic Performance with the Use of Noble-Metal-Decorated TiO2 Nanocrystals as Highly Active Catalysts for Aerobic Oxidation under Visible-Light Irradiation. Appl. Catal. B Environ. 2017, 210, 352–367. [Google Scholar] [CrossRef]
- Abbas, M.; Boumeddane, B.; Said, N.; Chikouche, A. Dish Stirling Technology: A 100 MW Solar Power Plant Using Hydrogen for Algeria. Int. J. Hydrogen Energy 2011, 36, 4305–4314. [Google Scholar] [CrossRef]
- Balghouthi, M.; Chahbani, M.H.; Guizani, A. Investigation of a Solar Cooling Installation in Tunisia. Appl. Energy 2012, 98, 138–148. [Google Scholar] [CrossRef]
- Augugliaro, V.; Baiocchi, C.; Prevot, A.B.; García-López, E.; Loddo, V.; Malato, S.; Marcí, G.; Palmisano, L.; Pazzi, M.; Pramauro, E. Azo-Dyes Photocatalytic Degradation in Aqueous Suspension of TiO2 under Solar Irradiation. Chemosphere 2002, 49, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Chafie, M.; Ben Aissa, M.F.; Guizani, A. Energetic End Exergetic Performance of a Parabolic Trough Collector Receiver: An Experimental Study. J. Clean. Prod. 2018, 171, 285–296. [Google Scholar] [CrossRef]
- Cao, F.; Wei, Q.; Liu, H.; Lu, N.; Zhao, L.; Guo, L. Development of the Direct Solar Photocatalytic Water Splitting System for Hydrogen Production in Northwest China: Design and Evaluation of Photoreactor. Renew. Energy 2018, 121, 153–163. [Google Scholar] [CrossRef]
- Basem, A.; Moawed, M.; Abbood, M.H.; El-Maghlany, W.M. The Energy and Exergy Analysis of a Combined Parabolic Solar Dish—Steam Power Plant. Renew. Energy Focus 2022, 41, 55–68. [Google Scholar] [CrossRef]
- Salamat, S.; Younesi, H.; Bahramifar, N. Synthesis of Magnetic Core-Shell Fe3O4@TiO2 Nanoparticles from Electric Arc Furnace Dust for Photocatalytic Degradation of Steel Mill Wastewater. RSC Adv. 2017, 7, 19391–19405. [Google Scholar] [CrossRef]
- Ung-Medina, F.; Caudillo-Flores, U.; Correa-González, J.C.; Maya-Yescas, R.; Chávez-Parga, M.D.C.; Cortés, J.A. Use of an Annular Non-Sleeve Photoreactor for Photocatalytic Dye Degradation: Study of Temperature and Light Intensity Effects. Environ. Prog. Sustain. Energy 2017, 36, 1083–1088. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Wu, Y.; Tu, W.; Wu, S.; Yuan, X.; Zeng, G.; Xu, Z.J.; Li, S.; Chew, J.W. Electrical Promotion of Spatially Photoinduced Charge Separation via Interfacial-Built-in Quasi-Alloying Effect in Hierarchical Zn2In2S5/Ti3C2(O, OH)x Hybrids toward Efficient Photocatalytic Hydrogen Evolution and Environmental Remediation. Appl. Catal. B Environ. 2019, 245, 290–301. [Google Scholar] [CrossRef]
- Liu, B.; Wu, H.; Parkin, I.P. New Insights into the Fundamental Principle of Semiconductor Photocatalysis. ACS Omega 2020, 5, 14847–14856. [Google Scholar] [CrossRef]
- Herrmann, J.M. Heterogeneous Photocatalysis: State of the Art and Present Applications In honor of Pr. R.L. Burwell Jr. (1912–2003), Former Head of Ipatieff Laboratories, Northwestern University, Evanston (Ill). Top. Catal. 2005, 34, 49–65. [Google Scholar] [CrossRef]
- Al-Soud, M.S.; Hrayshat, E.S. A 50 MW Concentrating Solar Power Plant for Jordan. J. Clean. Prod. 2009, 17, 625–635. [Google Scholar] [CrossRef]
- Balzar, A.; Stumpf, P.; Eckhoff, S.; Ackermann, H.; Grupp, M. A Solar Cooker Using Vacuum-Tube Collectors with Integrated Heat Pipes. Sol. Energy 1996, 58, 63–68. [Google Scholar] [CrossRef]
- Jafari Mosleh, H.; Mamouri, S.J.; Shafii, M.B.; Hakim Sima, A. A New Desalination System Using a Combination of Heat Pipe, Evacuated Tube and Parabolic through Collector. Energy Convers. Manag. 2015, 99, 141–150. [Google Scholar] [CrossRef]
- Abu-Hamdeh, N.H.; Alnefaie, K.A.; Almitani, K.H. Design and Performance Characteristics of Solar Adsorption Refrigeration System Using Parabolic Trough Collector: Experimental and Statistical Optimization Technique. Energy Convers. Manag. 2013, 74, 162–170. [Google Scholar] [CrossRef]
- Bigoni, R.; Kötzsch, S.; Sorlini, S.; Egli, T. Solar Water Disinfection by a Parabolic Trough Concentrator (PTC): Flow-Cytometric Analysis of Bacterial Inactivation. J. Clean. Prod. 2014, 67, 62–71. [Google Scholar] [CrossRef]
- Fernandez-Ibañez, P.; Malato, S.; Enea, O. Photoelectrochemical Reactors for the Solar Decontamination of Water. Catal. Today 1999, 54, 329–339. [Google Scholar] [CrossRef]
- García-Montaño, J.; Pérez-Estrada, L.; Oller, I.; Maldonado, M.I.; Torrades, F.; Peral, J. Pilot Plant Scale Reactive Dyes Degradation by Solar Photo-Fenton and Biological Processes. J. Photochem. Photobiol. A Chem. 2008, 195, 205–214. [Google Scholar] [CrossRef]
- Fernández-García, A.; Rojas, E.; Pérez, M.; Silva, R.; Hernández-Escobedo, Q.; Manzano-Agugliaro, F. A Parabolic-Trough Collector for Cleaner Industrial Process Heat. J. Clean. Prod. 2015, 89, 272–285. [Google Scholar] [CrossRef]
- Wu, S.Y.; Xiao, L.; Cao, Y.; Li, Y.R. A Parabolic Dish/AMTEC Solar Thermal Power System and Its Performance Evaluation. Appl. Energy 2010, 87, 452–462. [Google Scholar] [CrossRef]
- El-Kassaby, M.M. New Solar Cooker of Parabolic Square Dish: Design and Simulation. Renew. Energy 1991, 1, 59–65. [Google Scholar] [CrossRef]
- Grupp, M.; Balmer, M.; Beall, B.; Bergler, H.; Cieslok, J.; Hancock, D.; Schröder, G. On-Line Recording of Solar Cooker Use Rate by a Novel Metering Device: Prototype Description and Experimental Verification of Output Data. Sol. Energy 2009, 83, 276–279. [Google Scholar] [CrossRef]
- Badran, A.A.; Yousef, I.A.; Joudeh, N.K.; Hamad, R.; Al Halawa, H.; Hassouneh, H.K. Portable Solar Cooker and Water Heater. Energy Convers. Manag. 2010, 51, 1605–1609. [Google Scholar] [CrossRef]
- Srithar, K.; Rajaseenivasan, T.; Karthik, N.; Periyannan, M.; Gowtham, M. Stand Alone Triple Basin Solar Desalination System with Cover Cooling and Parabolic Dish Concentrator. Renew. Energy 2016, 90, 157–165. [Google Scholar] [CrossRef]
- Imran Khan, M.; Asfand, F.; Al-Ghamdi, S.G. Progress in Technology Advancements for next Generation Concentrated Solar Power Using Solid Particle Receivers. Sustain. Energy Technol. Assess. 2022, 54, 102813. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, Y.; Shen, Z.; Hu, M.; Yang, H. Concentrated Solar Power Tower Systems Coupled Locally with Spectrally Selective Coatings for Enhancement of Solar-Thermal Conversion and Economic Performance. Green Energy Resour. 2023, 1, 100001. [Google Scholar] [CrossRef]
- González-Mora, E.; Dolores Durán García, M. Methodology for an Opto-Geometric Optimization of a Linear Fresnel Reflector for Direct Steam Generation. Energies 2020, 13, 355. [Google Scholar] [CrossRef]
- Sebastián, A.; Abbas, R.; Valdés, M.; Casanova, J. Innovative Thermal Storage Strategies for Fresnel-Based Concentrating Solar Plants with East-West Orientation. Appl. Energy 2018, 230, 983–995. [Google Scholar] [CrossRef]
- Mills, D.R.; Morrison, G.L. Compact Linear Fresnel Reflector Solar Thermal Powerplants. Sol. Energy 2000, 68, 263–283. [Google Scholar] [CrossRef]
- Ochoa-Gutiérrez, K.S.; Tabares-Aguilar, E.; Mueses, M.Á.; Machuca-Martínez, F.; Li Puma, G. A Novel Prototype Offset Multi Tubular Photoreactor (OMTP) for Solar Photocatalytic Degradation of Water Contaminants. Chem. Eng. J. 2018, 341, 628–638. [Google Scholar] [CrossRef]
- Anderson, J.V.; Link, H.; Bohn, M.; Gupta, B. Development of Solar Detoxification Technology in the USA—An Introduction. Sol. Energy Mater. 1991, 24, 538–549. [Google Scholar] [CrossRef]
- Minero, C.; Pelizzetti, E.; Malato, S.; Blanco, J. Large Solar Plant Photocatalytic Water Decontamination: Degradation of Pentachlorophenol. Chemosphere 1993, 26, 2103–2119. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Richter, C.; Curco, D.; Gimenez, J. Low-Concentrating CPC Collectors for Photocatalytic Water Detoxification: Comparison with a Medium Concentrating Solar Collector. Water Sci. Technol. 1997, 35, 157–164. [Google Scholar] [CrossRef]
- Klare, M.; Scheen, J.; Vogelsang, K.; Jacobs, H.; Broekaert, J.A.C. Degradation of Short-Chain Alkyl- and Alkanolamines by TiO2- and Pt/TiO2-Assisted Photocatalysis. Chemosphere 2000, 41, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Negishi, N.; Takeuchi, K.; Matsuzawa, S. Degradation of Toluene and Acetaldehyde with Pt-Loaded TiO2 Catalyst and Parabolic Trough Concentrator. Sol. Energy 2004, 77, 543–552. [Google Scholar] [CrossRef]
- Bandala, E.R.; Arancibia-Bulnes, C.A.; Orozco, S.L.; Estrada, C.A. Solar Photoreactors Comparison Based on Oxalic Acid Photocatalytic Degradation. Sol. Energy 2004, 77, 503–512. [Google Scholar] [CrossRef]
- McLoughlin, O.A.; Kehoe, S.C.; McGuigan, K.G.; Duffy, E.F.; Al Touati, F.; Gernjak, W.; Oller Alberola, I.; Malato Rodríguez, S.; Gill, L.W. Solar Disinfection of Contaminated Water: A Comparison of Three Small-Scale Reactors. Sol. Energy 2004, 77, 657–664. [Google Scholar] [CrossRef]
- Barzegar, M.H.; Sabzehmeidani, M.M.; Ghaedi, M.; Avargani, V.M.; Moradi, Z.; Roy, V.A.L.; Heidari, H. S-Scheme Heterojunction g-C3N4/TiO2 with Enhanced Photocatalytic Activity for Degradation of a Binary Mixture of Cationic Dyes Using Solar Parabolic Trough Reactor. Chem. Eng. Res. Des. 2021, 174, 307–318. [Google Scholar] [CrossRef]
- Oyama, T.; Aoshima, A.; Horikoshi, S.; Hidaka, H.; Zhao, J.; Serpone, N. Solar Photocatalysis, Photodegradation of a Commercial Detergent in Aqueous TiO2 Dispersions under Sunlight Irradiation. Sol. Energy 2004, 77, 525–532. [Google Scholar] [CrossRef]
- Ma, R.; Su, H.; Sun, J.; Li, D.; Zhang, Z.; Wei, J. Concentrating Photo-Thermo-Organized Single-Atom and 2D-Raft Cu Catalyst for Full-Spectrum Solar Harmonic Conversion of Aqueous Urea and Urine into Hydrogen. Appl. Catal. B Environ. 2022, 315, 121493. [Google Scholar] [CrossRef]
- Vidal, A.; Díaz, A.I.; El Hraiki, A.; Romero, M.; Muguruza, I.; Senhaji, F.; González, J. Solar Photocatalysis for Detoxification and Disinfection of Contaminated Water: Pilot Plant Studies. Catal. Today 1999, 54, 283–290. [Google Scholar] [CrossRef]
- Kositzi, M.; Poulios, I.; Malato, S.; Caceres, J.; Campos, A. Solar Photocatalytic Treatment of Synthetic Municipal Wastewater. Water Res. 2004, 38, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.; Blanco, J.; Sichel, C.; Malato, S. Water Disinfection by Solar Photocatalysis Using Compound Parabolic Collectors. Catal. Today 2005, 101, 345–352. [Google Scholar] [CrossRef]
- Augugliaro, V.; García-López, E.; Loddo, V.; Malato-Rodríguez, S.; Maldonado, I.; Marcì, G.; Molinari, R.; Palmisano, L. Degradation of Lincomycin in Aqueous Medium: Coupling of Solar Photocatalysis and Membrane Separation. Sol. Energy 2005, 79, 402–408. [Google Scholar] [CrossRef]
- Villén, L.; Manjón, F.; García-Fresnadillo, D.; Orellana, G. Solar Water Disinfection by Photocatalytic Singlet Oxygen Production in Heterogeneous Medium. Appl. Catal. B Environ. 2006, 69, 1–9. [Google Scholar] [CrossRef]
- Sichel, C.; Tello, J.; de Cara, M.; Fernández-Ibáñez, P. Effect of UV Solar Intensity and Dose on the Photocatalytic Disinfection of Bacteria and Fungi. Catal. Today 2007, 129, 152–160. [Google Scholar] [CrossRef]
- Alrousan, D.M.A.; Polo-López, M.I.; Dunlop, P.S.M.; Fernández-Ibáñez, P.; Byrne, J.A. Solar Photocatalytic Disinfection of Water with Immobilised Titanium Dioxide in Re-Circulating Flow CPC Reactors. Appl. Catal. B Environ. 2012, 128, 126–134. [Google Scholar] [CrossRef]
- Quiñones, D.H.; Álvarez, P.M.; Rey, A.; Beltrán, F.J. Removal of Emerging Contaminants from Municipal WWTP Secondary Effluents by Solar Photocatalytic Ozonation. A Pilot-Scale Study. Sep. Purif. Technol. 2015, 149, 132–139. [Google Scholar] [CrossRef]
- Colina-Márquez, J.; Machuca-Martínez, F.; Puma, G.L. Modeling the Photocatalytic Mineralization in Water of Commercial Formulation of Estrogens 17-β Estradiol (E2) and Nomegestrol Acetate in Contraceptive Pills in a Solar Powered Compound Parabolic Collector. Molecules 2015, 20, 13354–13373. [Google Scholar] [CrossRef]
- Otálvaro-Marín, H.L.; Mueses, M.A.; Crittenden, J.C.; Machuca-Martinez, F. Solar Photoreactor Design by the Photon Path Length and Optimization of the Radiant Field in a TiO2-Based CPC Reactor. Chem. Eng. J. 2017, 315, 283–295. [Google Scholar] [CrossRef]
- Aguas, Y.; Hincapie, M.; Fernández-Ibáñez, P.; Polo-López, M.I. Solar Photocatalytic Disinfection of Agricultural Pathogenic Fungi (Curvularia Sp.) in Real Urban Wastewater. Sci. Total Environ. 2017, 607–608, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Haranaka-Funai, D.; Didier, F.; Giménez, J.; Marco, P.; Esplugas, S.; Machulek-Junior, A. Photocatalytic Treatment of Valproic Acid Sodium Salt with TiO2 in Different Experimental Devices: An Economic and Energetic Comparison. Chem. Eng. J. 2017, 327, 656–665. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Narciso-da-Rocha, C.; Polo-López, M.I.; Pastrana-Martínez, L.M.; Faria, J.L.; Manaia, C.M.; Fernández-Ibáñez, P.; Nunes, O.C.; Silva, A.M.T. Solar Treatment (H2O2, TiO2-P25 and GO-TiO2 Photocatalysis, Photo-Fenton) of Organic Micropollutants, Human Pathogen Indicators, Antibiotic Resistant Bacteria and Related Genes in Urban Wastewater. Water Res. 2018, 135, 195–206. [Google Scholar] [CrossRef]
- López, N.; Marco, P.; Giménez, J.; Esplugas, S. Photocatalytic Diphenhydramine Degradation under Different Radiation Sources: Kinetic Studies and Energetic Comparison. Appl. Catal. B Environ. 2018, 220, 497–505. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Solar Reclamation of Wastewater Effluent Polluted with Bisphenols, Phthalates and Parabens by Photocatalytic Treatment with TiO2/Na2S2O8 at Pilot Plant Scale. Chemosphere 2018, 212, 95–104. [Google Scholar] [CrossRef]
- Luna-Sanguino, G.; Ruíz-Delgado, A.; Tolosana-Moranchel, A.; Pascual, L.; Malato, S.; Bahamonde, A.; Faraldos, M. Solar Photocatalytic Degradation of Pesticides over TiO2-RGO Nanocomposites at Pilot Plant Scale. Sci. Total Environ. 2020, 737, 140286. [Google Scholar] [CrossRef]
- Zheng, Q.; Aiello, A.; Choi, Y.S.; Tarr, K.; Shen, H.; Durkin, D.P.; Shuai, D. 3D Printed Photoreactor with Immobilized Graphitic Carbon Nitride: A Sustainable Platform for Solar Water Purification. J. Hazard. Mater. 2020, 399, 123097. [Google Scholar] [CrossRef]
- Minero, C.; Pelizzetti, E.; Malato, S.; Blanco, J. Large Solar Plant Photocatalytic Water Decontamination: Degradation of Atrazine. Sol. Energy 1996, 56, 411–419. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Vidal, A.; Richter, C. Photocatalysis with Solar Energy at a Pilot-Plant Scale: An Overview. Appl. Catal. B Environ. 2002, 37, 1–15. [Google Scholar] [CrossRef]
- Cao, F.; Pang, J.; Gu, X.; Wang, M.; Shangguan, Y. Performance Simulation of Solar Trough Concentrators: Optical and Thermal Comparisons. Energies 2023, 16, 1673. [Google Scholar] [CrossRef]
- Mussard, M.; Nydal, O.J. Charging of a Heat Storage Coupled with a Low-Cost Small-Scale Solar Parabolic Trough for Cooking Purposes. Sol. Energy 2013, 95, 144–154. [Google Scholar] [CrossRef]
- Stanek, B.; Węcel, D.; Bartela, Ł.; Rulik, S. Solar Tracker Error Impact on Linear Absorbers Efficiency in Parabolic Trough Collector—Optical and Thermodynamic Study. Renew. Energy 2022, 196, 598–609. [Google Scholar] [CrossRef]
- Beltran, R.; Velazquez, N.; Espericueta, A.C.; Sauceda, D.; Perez, G. Mathematical Model for the Study and Design of a Solar Dish Collector with Cavity Receiver for Its Application in Stirling Engines. J. Mech. Sci. Technol. 2012, 26, 3311–3321. [Google Scholar] [CrossRef]
- Nepveu, F.; Ferriere, A.; Bataille, F. Thermal Model of a Dish/Stirling Systems. Sol. Energy 2009, 83, 81–89. [Google Scholar] [CrossRef]
- El Ouederni, A.R.; Salah, M.B.; Askri, F.; Nasrallah, M.B.; Aloui, F. Experimental Study of a Parabolic Solar Concentrator. J. Renew. Energ. 2009, 12, 395–404. [Google Scholar]
- Abbas, R.; Montes, M.J.; Piera, M.; Martínez-Val, J.M. Solar Radiation Concentration Features in Linear Fresnel Reflector Arrays. Energy Convers. Manag. 2012, 54, 133–144. [Google Scholar] [CrossRef]
- Geyer, M.; Lüpfert, E.; Osuna, R.; Nava, P.; Langenkamp, J.; Mandelberg, E. EUROTROUGH-Parabolic Trough Collector Developed for Cost Efficient Solar Power Generation. In Proceedings of the 11th International Symposium on Concentrating Solar Power and Chemical Energy Technologies, Zurich, Switzerland, 4–6 September 2002; Volume 7. [Google Scholar]
- Sharma, A.K.; Sharma, C.; Mullick, S.C.; Kandpal, T.C. GHG Mitigation Potential of Solar Industrial Process Heating in Producing Cotton Based Textiles in India. J. Clean. Prod. 2017, 145, 74–84. [Google Scholar] [CrossRef]
- Palenzuela, P.; Zaragoza, G.; Alarcón-Padilla, D.C.; Guillén, E.; Ibarra, M.; Blanco, J. Assessment of Different Configurations for Combined Parabolic-Trough (PT) Solar Power and Desalination Plants in Arid Regions. Energy 2011, 36, 4950–4958. [Google Scholar] [CrossRef]
- Peiró, G.; Prieto, C.; Gasia, J.; Jové, A.; Miró, L.; Cabeza, L.F. Two-Tank Molten Salts Thermal Energy Storage System for Solar Power Plants at Pilot Plant Scale: Lessons Learnt and Recommendations for Its Design, Start-up and Operation. Renew. Energy 2018, 121, 236–248. [Google Scholar] [CrossRef]
- de Risi, A.; Milanese, M.; Laforgia, D. Modelling and Optimization of Transparent Parabolic Trough Collector Based on Gas-Phase Nanofluids. Renew. Energy 2013, 58, 134–139. [Google Scholar] [CrossRef]
- Linares, J.I.; Montes, M.J.; Cantizano, A.; Sánchez, C. A Novel Supercritical CO2 Recompression Brayton Power Cycle for Power Tower Concentrating Solar Plants. Appl. Energy 2020, 263, 114644. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, H.; Lu, H.; Yang, Z.; Guo, H. Numerical Investigation of Heat Transfer and Flow Characteristics of Supercritical CO2 in Solar Tower Microchannel Receivers at High Temperature. Energies 2023, 16, 6445. [Google Scholar] [CrossRef]
- Pino, F.J.; Caro, R.; Rosa, F.; Guerra, J. Experimental Validation of an Optical and Thermal Model of a Linear Fresnel Collector System. Appl. Therm. Eng. 2013, 50, 1463–1471. [Google Scholar] [CrossRef]
- Beltagy, H.; Semmar, D.; Lehaut, C.; Said, N. Theoretical and Experimental Performance Analysis of a Fresnel Type Solar Concentrator. Renew. Energy 2017, 101, 782–793. [Google Scholar] [CrossRef]
- Mills, D.R.; Morrison, G.L. Modelling Study for Compact Fresnel Reflector Power Plant. Le J. De Phys. IV 1999, 9, Pr3-159–Pr3-165. [Google Scholar] [CrossRef]









| Thematic Axis of Reactor | Dimension of Sunlight Reflector | Reactant Volume | Temperature | Irradiance | Deployed Location | Photocatalyst | Treatment Target | Timeline | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PTR | - | - | - | - | U.S. (latitude: 38.6° N) | TiO2 | Groundwater | 1991 | [74] |
| Width: 1.8 m; Length: 4.5 m | - | - | - | Spain (latitude: 37.4° N) | TiO2 | Pentachlorophenol | 1993 | [75] | |
| Width: 1.8 m; Length: 4.5 m | 220 L | - | - | Spain (latitude: 37.4° N) | TiO2 | Atrazine | 1996 | [75] | |
| 29.1 m2 | 260 L | - | - | Spain (latitude: 37.4° N) | TiO2 | 2,4-Dichlorophenol | 1997 | [76] | |
| - | 80 L | - | - | Germany (latitude: 50.6° N) | TiO2 | C2H5NH2 and (C2H5)2NH | 2000 | [77] | |
| Width: 1 m; Length: 1 m | - | 200–250 °C | - | Japan (latitude: 36° N) | Pt-TiO2 | Toluene, acetaldehyde | 2004 | [78] | |
| 0.72 m2 | 10 L | - | - | Mexico (latitude: 19° N) | TiO2 | Oxalic acid | 2004 | [79] | |
| 0.042 m2 | 1 L | <38 °C | - | Spain (latitude: 37° N) | TiO2 | Escherichia coli | 2004 | [80] | |
| Width: 0.5 m; Length: 1.4 m | - | - | - | Iran (latitude: 22.4° N) | g-C3N4/TiO2 | Methylene blue and rhodamine b | 2021 | [81] | |
| PDR | 0.785 m2 | 3 L | 30–58 °C | 10–35 W/m2 (UV) | Japan (latitude: 35.4° N) | TiO2 | Detergent | 2004 | [82] |
| LFR | Width: 0.055 m; Length: 0.23 m | 1 L | ≤52 °C | 24–40 W/m2 (UV) | Japan (latitude: 36.1° N) | TiO2 | Rhodamine b, amoxicillin, Escherichia coli | 2022 | [7] |
| 0.077 m2 | 1 L | 40 °C | 23.6 W/m2 (UV) | Japan (latitude: 36.1° N) | TiO2 | Chlorella vulgaris | 2023 | [1] | |
| FL | - | 0.05 L | 93 °C | 15,000 W/m2 | China (latitude: 34.5° N) | Cu-TiO2−x | Urea, urine | 2022 | [83] |
| V-groove | 0.72 m2 | 10 L | - | - | Mexico (latitude: 19° N) | TiO2 | Oxalic acid | 2004 | [79] |
| 0.057 m2 | 1 L | <38 °C | - | Spain (latitude: 37° N) | TiO2 | Escherichia coli | 2004 | [80] | |
| CPC | 8.9 m2 | 247 L | - | - | Spain (latitude: 37.4° N) | TiO2 | 2,4-Dichlorophenol | 1997 | [76] |
| 4.5 m2 | 25 L | <40 °C | 15–25 W/m2 (UV) | Spain (latitude: 43° N) | TiO2 | Escherichia coli, Enterococcus faecalis | 1999 | [84] | |
| 20–35 W/m2 (UV) | Morocco (latitude: 34° N) | ||||||||
| 3.08 m2 | 39 L | - | - | Spain (latitude: 37° N) | TiO2 | Azo-dyes | 2002 | [46] | |
| 0.72 m2 | 10 L | - | - | Mexico (latitude: 19° N) | TiO2 | Oxalic acid | 2004 | [79] | |
| 0.057 m2 | 1 L | <38 °C | - | Spain (latitude: 37° N) | TiO2 | Escherichia coli | 2004 | [80] | |
| 3.08 m2 | 35 L | <45 °C | - | Spain (latitude: 37° N) | TiO2 | Peptone, meat extract, urea, etc. | 2004 | [85] | |
| 0.25 m2 | 11 L | 15.8–36.5 °C | - | Spain (latitude: 37.4° N) | TiO2 | Escherichia coli | 2005 | [86] | |
| 3.08 m2 | 39 L | - | - | Spain (latitude: 37° N) | TiO2 | Lincomycin | 2005 | [87] | |
| 0.8 m2 | 17.5 L | 25–48 °C | 400 ± 25 W/m2 | Spain (latitude: 40° N) | Ruthenium (II) tris–chelate complex | Escherichia coli, Escherichia faecalis | 2006 | [88] | |
| 0.4 m2 | 14 L | <30 °C | 16.5 W/m2 (UV) | Spain (latitude: 37.09° N) | TiO2 | Escherichia coli, Fusarium solani, Fusarium anthophilum | 2007 | [89] | |
| 0.2 m2 | 7 L | <32 °C | 2.8–7.2 W/m2 (UV) | Spain (latitude: 37° N) | TiO2 | Escherichia coli | 2012 | [90] | |
| 0.25 m2 | 5–7 L | - | 35–46 W/m2 (UV) | Spain (latitude: 38.5° N) | TiO2 | Acetaminophen, antipyrine, bisphenol A, caffeine, metoprolol and testosterone | 2015 | [91] | |
| Length: 1.2 m | 40 L | - | 30 W/m2 (UV) | Colombia (latitude: 3.5° N) | TiO2 | 17-β estradiol and nomegestrol acetate | 2015 | [92] | |
| Width: 0.207 m | - | - | - | Colombia (latitude: 3.4° N) | TiO2 | 2017 | [93] | ||
| 1 m2 | 20 L | 26.1–38.2 °C | 15.7–33.1 W/m2 (UV) | Spain (latitude: 37.09° N) | TiO2 | Curvularia sp. | 2017 | [94] | |
| 0.228 m2 | 5 L | 37 °C | - | Spain (latitude: 41.4° N) | TiO2 | Valproic acid sodium salt | 2017 | [95] | |
| 1 m2 | 15 L | 16.6–43.2 °C | 40 W/m2 (UV) | Spain (latitude: 37.84° N) | TiO2 | Pseudomonas, Rheinheimera and Methylotenera | 2018 | [96] | |
| 0.228 m2 | 5 L | 30 ± 5 °C | 12.45–49.78 W/m2 (UV) | Spain (latitude: 41.4° N) | TiO2 | Diphenhydramine hydrochloride | 2018 | [97] | |
| - | 100 L | - | - | Spain (latitude: 37.6° N) | TiO2 | Endocrine disruptors | 2018 | [98] | |
| 2.56 m2 | 11.4 L | - | ≈2083 W/m2 | China (latitude: 34.5° N) | Cd0.5Zn0.5S | Water splitting | 2018 | [48] | |
| 1.74 m2 | 36 L | 33 °C | 1573 W/m2 | Colombia (latitude: 10.2° N) | TiO2 | Methylene blue, dichloroacetic acid, 4- Chlorophenol, phenol | 2018 | [73] | |
| 1.74 m2 | 65 L | 1259 W/m2 | |||||||
| 3.2 m2 | 35 L | - | 30 W/m2 (UV) | Spain (latitude: 37.09° N) | TiO2-rGO | Methomyl, Isoproturon, Alachlor | 2020 | [99] | |
| Width: 0.75 m | - | - | - | U.S. (latitude: 38.5° N) | g-C3N4/chitosan | Atrazine, phenol, sulfamethoxazole, carbamazepine, Escherichia coli | 2020 | [100] | |
| Width: 3.6 m | |||||||||
| 1 m2 | 20 L | 12–20 °C | 664–1018 W/m2 | Chile (latitude: 37.1° S) | Cu@C | Tartrazine | 2022 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, N.; An, G. Review of Concentrated Solar Power Technology Applications in Photocatalytic Water Purification and Energy Conversion: Overview, Challenges and Future Directions. Energies 2024, 17, 463. https://doi.org/10.3390/en17020463
Zhang C, Li N, An G. Review of Concentrated Solar Power Technology Applications in Photocatalytic Water Purification and Energy Conversion: Overview, Challenges and Future Directions. Energies. 2024; 17(2):463. https://doi.org/10.3390/en17020463
Chicago/Turabian StyleZhang, Cheng, Na Li, and Guangqi An. 2024. "Review of Concentrated Solar Power Technology Applications in Photocatalytic Water Purification and Energy Conversion: Overview, Challenges and Future Directions" Energies 17, no. 2: 463. https://doi.org/10.3390/en17020463
APA StyleZhang, C., Li, N., & An, G. (2024). Review of Concentrated Solar Power Technology Applications in Photocatalytic Water Purification and Energy Conversion: Overview, Challenges and Future Directions. Energies, 17(2), 463. https://doi.org/10.3390/en17020463