Single-Chamber Solid Oxide Fuel Cell Technology—From Its Origins to Today’s State of the Art
Abstract
:1. Introduction
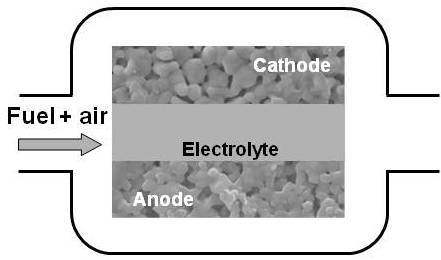
2. Single-Chamber Solid Oxide Fuel Cells

| Advantages | Challenges |
|---|---|
|
|
3. Origins of SC-SOFCs
3.1. Early works on single-chamber fuel cells
3.2. Dyer’s room-temperature single-chamber fuel cell

3.3. Development of SC-SOFCs
4. Single-Chamber Operation
4.1. Background on SOFCs
4.2. Working principles of SC-SOFCs

4.3. Fuel-air mixtures
4.4. Electrode selectivity and catalytic activity
4.5. Fuel utilization and fuel cell efficiency
4.6. Heat production
4.7. Testing chamber design
4.8. Current collection
4.9. Flammability and explosion limits of methane-air mixtures


5. Development of SC-SOFCs

5.1. Planar electrolyte-supported SC-SOFCs
5.1.1. Early developments – The work of Hibino et al.
5.1.2. Confirmation of the feasibility of SC-SOFCs
5.1.3. Materials
Anode materials
Cathode materials
Electrolyte materials
5.1.4. Effect of testing conditions
5.1.5. Reduction of the operating temperature
5.1.6. Cell initialization
5.1.7. Cell stacks
5.1.8. Summary of planar electrolyte-supported SC-SOFCs
| Reference | Year | Electrolyte | Electrolyte thickness (mm) | Anode | Cathode | Gas mixture | Tfurnace ( °C) | OCV (V) | Pmax (mW·cm−2) |
|---|---|---|---|---|---|---|---|---|---|
| [48] | 1993 | YSZ | 0.5 | Ni-YSZ | Au | CH4-air Rmix = 2 | 950 | 0.35 | 2.36 |
| [108,109]/ [8]/[111] | 1994/ 1995/1998 | YSZ | 0.5 | Pt | Au | CH4-air Rmix = 2 | 950 | 0.2 | 0.2 |
| SrCe0.95Yb0.05O3-α | 0.66 | 21 | |||||||
| [8,68,110]/ [111] | 1995/1998 | BaCe0.8Y0.2O3-α | 0.5 | Pt | Au | CH4-air Rmix = 2 | 950 | 0.77 | 170 |
| [8]/[111] | 1995/1998 | (CeO2)0.9(SmO1.5)0.1 | 0.5 | Pt | Au | CH4-air Rmix = 2 | 950 | 0.3 | |
| [112] | 1996 | TiO2-doped YSZ | 0.5 | Pt | Au | CH4-air Rmix = 2 | 950 | 0.47 | 3 |
| [113] | 1997 | Pr6O11-doped YSZ | 0.5 | Pt | Au | CH4-air Rmix = 2 | 950 | 0.7 | 34 |
| [16] | 1997 | YSZ | 0.06 | Pt | Au | CH4-air | 700 | 0.8 | 6 |
| [7] | 1998 | YSZ | Pt | Au | CH4-air Rmix = 2 | 950 | 0.2 | ||
| Pr-doped YSZ | 2.5–3 | ||||||||
| Pr-coated YSZ | 2.4 | ||||||||
| SrCe0.95Y0.05O3-α | 4 | ||||||||
| [114] | 1999 | YSZ | 0.5 | Pt-MnO2 | Au-MnO2 | CH4-air Rmix = 1 | 950 | 0.6 | 48 |
| MnO2-doped YSZ | 52 | ||||||||
| [6] | 1999 | YSZ | 0.5 | Pt | Au | CH4-air Rmix = 1 | 950 | 0.5 | 0.57 |
| Ni | LSM | 0.8 | 121 | ||||||
| Ni-GDC | LSM-MnO2 | 0.83 | 162 | ||||||
| [115] | 1999 | LSGM | 0.5 | Ni | LSM | CH4-air Rmix = 1 | 950 | 0.8 | 150 |
| [69] | 2000 | YSZ | 0.3 | Ni-GDC | LSM-MnO2 | CH4-air Rmix = 1 | 950 | 0.8 | 204 |
| [52] | 2000 | SDC | 0.15 | Ni-SDC | SSC | C2H6-air Rmix = 1 | 500 | 0.9 | 400 |
| LSGM | 0.5 | ||||||||
| YSZ | |||||||||
| [117] | 2000 | MnO2-doped YSZ | 0.3 | Ni-GDC | LSM-MnO2 | CH4-air Rmix = 1 | 950 | 0.8 | 256 |
| [55] | 2000 | LSGM | 0.5 | Ni-SDC | SSC | CH4-air Rmix = 1 | 800 | 0.9 | 450 |
| YSZ | 700 | 0.9 | 125 | ||||||
| SDC | 0.72 | 150 | |||||||
| [82] | 2000 | (Y2O3)0.04(Sc2O3)0.06 -(ZrO2)0.9 | 0.7 | Ni-YSZ-CeO2 | LSM | CH4-air Rmix = 1.9 | 600 | 0.8 | 8.5 |
| [137] | 2001 | YSZ | 0.5 | Ni-SDC | SSC | C2H6-air Rmix = 1 | 500 | 0.9 | |
| SDC | 0.15 | 450 | 280 | ||||||
| [93] | 2001 | SDC | 0.15 | Ni-SDC | SSC | C4H10-air | 450 | 0.9 | 245 |
| [123] | 2001 | CZI10 | 1 | Ni-CZI10 | Pt | CH4-air | 800 | 0.4 | 0.01 |
| [80] | 2002 | SDC | 0.15 | Ni-SDC | SSC | CH4-air Rmix = 1 | 550 | 0.82 | 644 |
| [125] | 2003 | YSZ | 0.1 | Ni-YSZ | LSM | C3H8-air Rmix = 1/1.8 | 600 | 0.9 | 4 |
| SSC | 14 | ||||||||
| LSCF | 16 | ||||||||
| [53] | 2003 | YSZ | Ni-YSZ | LSM | C3H8-air Rmix = 1/1.8 | 600 | 4.25 | ||
| [126]/[127] | 2003/2005 | SDC | Ni-SDC | LSCF-SDC | C3H8-air Rmix = 1/1.8 | 600 | 0.8 | 140 | |
| [128] | 2004 | SDC | 0.5 | Ni-SDC | LSCF-SDC | C3H8-air Rmix = 1/1.8 | 650 | 0.8 | 210 |
| [26,27] | 2004 | YSZ | 0.2 | Ni-YSZ | LSM | CH4-air Rmix = 2 | 800 | 1.02 | 85 |
| [97] | 2004 | SDC | 0.8 | Ni-SDC | SSC | C3H8-air Rmix = 1/1.9 | 600 | 0.7 | 18 |
| [141]/[127] | 2004/2005 | SDC | 0.5 | Ni-SDC | SDC-LSCF | C3H8-air Rmix = 1/1.8 | 575 | 0.8 | 110 |
| [118] | 2004 | YSZ | 0.1 | Ni-YSZ | LSCF | C3H8-air Rmix = 1/1.8 | 550 | 0.87 | |
| Ni-CGO | 600 | 0.86 | |||||||
| Ni-CSO | 600-635 | 0.84 | |||||||
| [99,100]/[101] | 2004/2005 | YSZ | 0.2 | Ni-CGO | SSC | CH4-air Rmix = 1.7 | 600 | 0.68 | 468 |
| [138] | 2005 | YSZ | 0.5 | Ni-LSCM | SSC | CH4-air Rmix = 1.25 | 800 | 0.6 | 5 |
| BaLaIn2O5.5 | 38 | ||||||||
| [134] | 2005 | YSZ | 0.2 | Ni-YSZ | Nd1.95NiO4+δ | CH4-air Rmix = 2.6 | 700 | 1 | 55 |
| LSM | 34 | ||||||||
| [135] | 2006 | CGO | 0.5 | Ni-CGO | LSCO-CGO | C3H8-air Rmix = 0.58 | 625 | 0.85 | 65 |
| [129] | 2006 | SDC | Ni-YDC | LSCF | CH4-air Rmix = 1 | 800 | 0.7 | 186 | |
| LSM | 0.46 | 30 | |||||||
| [139] | 2006 | CGO | 1 | Ni-CGO | SSC | CH4-air Rmix = 2 | 733 (Tanode) | 0.85 | 70 |
| [119] | 2007 | YSZ | 0.5 | Ni-ZrO2-CeO2 | LSM | CH4-air Rmix = 1 | 950 | 1.1 | 53 |
| [121]/[81] | 2007/2009 | SDC | 0.2 | Ni-Pd-SDC | SSC | CH4-air Rmix = 2 | 600 | 0.8 | 97 |
| [92]/[94] | 2007/2009 | YSZ | 0.5 | Ni-YSZ | LSM-YSZ | CH4-air Rmix = 1 | 700 | 0.92 | 114 |
| [124] | 2008 | YSZ | 0.4 | Ni-YSZ | LSM | CH4-air Rmix = 2 | 690 | 1.14 | |
| [142,143] | 2008 | YSZ | 0.4 | Ni-CSO | LSM | Hydrocarbon-air | 800 | 1.1 | 19 |
| [136] | 2009 | CGO | 0.2 | Ni-CGO | LSCO-CGO | CH4-air Rmix = 0.9 | 740 | 0.7 | 280 |
| [133] | 2009 | SDC | 0.3 | Ni-SDC | Pr0.7Sr0.3Fe0.8Co0.2O3-δ | CH4-air Rmix = 2 | 600 | 0.73 | 65 |
5.2. Planar anode-supported SC-SOFCs
5.2.1. Materials
Anode materials
Cathode materials
Electrolyte materials
5.2.2. Effect of operating conditions
5.2.3. Low temperature operation
5.2.4. Liquid fuels
5.2.5. Thermally self-sustaining SC-SOFCs
5.2.6. Cell initialization
5.2.7. Cell stacks
5.2.8. Comparison of electrolyte- and anode supported SC-SOFCs
5.2.9. Summary of planar anode supported SC-SOFCs
5.3. Fully porous SC-SOFCs
| Reference | Year | Electrolyte | Electrolyte thickness (μm) | Anode | Cathode | Gas mixture | Tfurnace ( °C) | OCV (V) | Pmax (mW·cm−2) |
|---|---|---|---|---|---|---|---|---|---|
| [53] | 2003 | YSZ | 10 | Ni-YSZ | LSM | C3H8-air Rmix = 1/1.8 | 475 | 0.88 | 3.4 |
| [28] | 2004 | YSZ | 2 | Ni-YSZ | LSCF | CH4-air Rmix = 1 | 750 | 0.85 | 120 |
| [154] | 2004 | SDC | 20 | Ni-SDC | BSCF-SDC | C3H8-air Rmix = 0.44 | 500 | 0.68 | 440 |
| [27] | 2004 | YSZ | 10 | Ni-YSZ | LSM | CH4-air Rmix = 0.88 | 800 | 0.84 | 360 |
| [54] | 2004 | SDC | 10-20 | Ni-SDC | SSC-SDC | C3H8-air Rmix = 0.4 | 525 | 0.68 | 210 |
| [79] | 2004 | YSZ | Ni-YSZ | LSCF-GDC | C3H8-air Rmix = 0.6 | 750 | 1 | 700 | |
| [25] | 2005 | SDC | 20 | Ni-SDC-Ru-CeO2 | BSCF-SDC | C3H8-air Rmix = 0.44 | 580 (Tcell) | 0.7 | 247 |
| [9] | 2005 | SDC | 20 | Ni-Al-SDC | LSM-SDC | CH4-air Rmix = 0.65 | 700 | 0.84 | 305 |
| [146] | 2005 | GDC | 15 | Ni-SDC | SSC | C4H10-air Rmix = 2 | 300 | 0.9 | 133 |
| Ni-SDC-Ru | 0.92 | 176 | |||||||
| Ni-SDC-Pd | 0.8 | 110 | |||||||
| [76] | 2005 | YSZ | 10 | Ni-YSZ | LSM | CH4-air-H2O Rmix = 1.2 | 700 | 0.95 | 90 |
| [12] | 2006 | YSZ | 30 | Ni-YSZ-SDC | LSM | CH4-air Rmix = 1.67 | 700 | 1.1 | 280 |
| [96] | 2006 | YSZ | 10 | Ni-YSZ | LSM | CH4-air Rmix = 2 | 800 | 1.1 | 260 |
| [145,161] | 2006 | SDC | 15-20 | Ni-SDC | BSCF-SDC | CH4-air Rmix = 1.16 | 650 | 0.7 | 760 |
| [163] | 2006 | GDC-BCY | 26 | Ni-SDC | SSC | CH4-air Rmix = 2 | 500 | 0.9 | 302 |
| [144] | 2006 | YSZ | 8 | Ni-YSZ | LSM | CH4-air | 700 | 1 | 398 |
| [140] | 2006 | YSZ | 10 | Ni-YSZ | LSM | CH4-air Rmix = 2 | 700 | 1.03 | 225 |
| [153] | 2007 | CGO | 15 | Ni-CGO | LSCO-CGO | C3H8-air Rmix = 0.11 | 500 | 0.7 | 38 |
| [56] | 2007 | GDC | 15 | Ni-SDC | SSC | C4H10-air Rmix = 0.5 | 300 | 0.9 | 59 |
| Ni-SDC-Ru | Dimethyl ether-air | 0.75 | 64 | ||||||
| Ni-SDC-Cu/Zn/Al | C2H5OH-air | 0.8 | 117 | ||||||
| [57] | 2007 | YSZ | 8 | Ni-YSZ | LSM | CH4-air Rmix = 1 | 600 | 0.94 | 220 |
| [88] | 2007 | YSZ | 15 | Ni-YSZ | LSM-SDC | CH4-air Rmix = 1 | 650 | 0.96 | 300 |
| [159,160] | 2007 | SDC | 20 | Ni-SDC | SSC-SDC | C4H10-air Rmix = 0.5 | 550 | 0.6 | 250 |
| BSCF-SDC | C3H8-air Rmix = 1/1.54 | 0.68 | 420 | ||||||
| [156]/[167] | 2007/2009 | YSZ | 8 | Ni-YSZ | LSM | CH4-air Rmix = 1.5 | 750 | 0.92 | 125 |
| LSM-SDC | 0.97 | 416 | |||||||
| [162] | 2008 | SDC | 20-30 | Ni-SDC | BSCF-SDC | CH4-air Rmix = 1 | 600 | 0.75 | 570 |
| [166] | 2009 | YSZ | 15 | Ni-YSZ | LSM-SDC | CH4-air Rmix = 1 | 700 | 0.9 | 150 |
| [155] | 2009 | ScSZ | 20-30 | Ni-ScSZ | LSM | CH4-air Rmix = 1.3 | 850 | 0.95 | 275 |
| LSSM | 0.9 | 250 | |||||||
| [83] | 2009 | ScSZ | 30 | Ni-ScSZ-Ru-CeO2 | LSM | CH4-air Rmix = 1.3 | 850 | 0.95 | 327 |

5.3.1. Flow-by fully-porous SC-SOFCs
5.3.2. Flow-through fully-porous SC-SOFCs
5.4. SC-SOFCs with coplanar electrodes

5.4.1. Development of SC-SOFCs with coplanar electrodes
5.4.2. Performance considerations

5.4.3. Testing conditions
5.4.4. Microfabrication techniques
5.4.5. SC-SOFCs with interdigitated electrode designs
5.4.6. SC-SOFCs with arbitrary electrode designs
5.4.7. Cell stacks
5.4.8. Summary of SC-SOFCs with coplanar electrodes
5.5. Micro-tubular SC-SOFCs and other cell configurations
| Ref. | Year | Fabrication technique | Gap size d (mm) | Electrode width w (mm) | Electrolyte | Anode | Cathode | Tfurnace ( °C) | Gas mixture | OCV (V) | Pmax or Imax (for smallest d and w) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [36] | 1981 | Painted* | 0.3–0.4 | 0.5 | Alumina | Pt-Al2O3 | SrRuO3-Al2O3 | 21 | H2-O2-N2 | 0.67 | 3 μA |
| [107] | 1995 | Smeared* | 0.5–5 | BaCe0.8Gd0.2O3-α | Pd | Au | 950 | CH4-air Rmix = 2 | 0.7 | 24 mA | |
| [174] | 1996 | Smeared* | 0.5–5 | 1–4 | BaCe0.8Gd0.2O3-α | Pd | Au | 950 | CH4-air Rmix = 2 | 0.7 | 24 mA |
| [69] | 2000 | Smeared* | 1–4 | YSZ | Ni-GDC | LSM-MnO2 | 950 | CH4-air Rmix = 1 | 0.8 | 102 mW·cm−2 | |
| [117] | 2000 | Smeared* | 0.5–3 | YSZ-MnO2 | Ni-GDC | LSM-MnO2 | 950 | CH4-air Rmix = 1 | 0.8 | 143 mW·cm−2 | |
| [137] | 2001 | Smeared* | 0.5 | YSZ | Ni | LSM | 950 | CH4-air | 0.8 | 140 mW·cm−2 | |
| [147] | 2002 | Smeared* | 0.5-3 | 0.5–1 | SDC | Ni-SDC-PdO | SSC | 600 | C4H10-air Rmix = 0.6 | 0.8 | 245 mW·cm−2 |
| [147] | 2002 | Smeared* | 1 | 1 | YSZ | Ni-SDC | SSC | 600 | C2H6-air Rmix = 1.1 | 0.97 | 20 mW·cm−2 |
| [147] | 2002 | Smeared* | 1 | 1 | LSGM | Ni-SDC | SSC | 600 | C2H6-air Rmix = 1.1 | 0.92 | 50 mW·cm−2 |
| [98] | 2005 | Screen-printing | 1 | 6 | YSZ | Ni-YSZ | LSM | 800 | CH4-air Rmix = 1.5 | 0.6 | 1.2 mW·cm−2 |
| [198] | 2006 | Screen-printing | 1 | 6 | YSZ | Ni-YSZ | LSM | 770 | CH4-air Rmix = 2 | 0.5 | 1 mW·cm−2 |
| [198] | 2006 | Screen-printing | 2 | 1 | YSZ | Ni-YSZ | LSM | 760 | CH4-air Rmix = 2 | 0.6 | 1 mW·cm−2 |
| [184,185] | 2006 | Direct- writing | 0.255–0.783 | 0.6 | YSZ | Ni-GDC-Pd | LSM | 900 | CH4-air Rmix = 3.75 | 0.8 | 101 mW·cm−2 |
| [180] | 2007 | Direct- writing | 0.255–0.783 | 0.6 | YSZ | Ni-GDC-Pd | LSM-GDC | 900 | CH4-air + 3% H2O Rmix = 3.75 | 0.8 | 101 mW·cm−2 |
| [150] | 2007 | Tape casting | 0.2–1 | 0.5–4 | YSZ | Ni-YSZ | LSM | 800 | CH4-air Rmix = 2 | 0.9 | 40 mW·cm−2 |
| [103] | 2007 | Screen-printing | 1.2 | 0.5 | GDC | Ni-GDC | SSC | 600 | CH4-air Rmix = 3.2 | 0.8 | 1.5 mW·cm−2 |
| [186] | 2007 | Sputtering, lithography | 0.005 | 0.015 | YSZ | Pt | Au | 400 | CH4-air Rmix = 1 | 0.38 | |
| [171] | 2008 | Smeared* | 1–1.2 | 1 | YSZ SDC | Pt | Pt LSM | 600–1,000 | H2-O2 | 0–0.7 | |
| [194] | 2008 | 0.5 | Al2O3 | Pt | Pt | Room T | H2-O2 | 0.54 | |||
| [151] | 2009 | Direct- writing | 0.25 | 0.093–1.38 | YSZ | Ni-YSZ | LSM | 700 | CH4-air Rmix = 2 | 0.898 | 10.5 mW·cm−2 |
| Ref. | Year | Fabrication technique | Gap size d (mm) | Electrode width w (mm) | Electrolyte | Anode | Cathode | Tfurnace ( °C) | Gas mixture | OCV (V) | Pmax (mW·cm−2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [172] | 2005 | Photoresist molding | 0.02 | 0.02 | GDC | Ni-GDC | SSC | 500 | CH4-air | 0.2 | 67 |
| [12] | 2006 | Screen-printing | 0.5 | SDC | Ni-SDC | LSM | 700 | CH4-air Rmix = 1 | 0.8 | 40 | |
| [12] | 2006 | Screen-printing | 0.5 | YSZ | Ni-SDC | LSM | 700 | CH4-air Rmix = 1 | 0.9 | ||
| [189] | 2006 | Microfluidic lithography | 0.05 | 0.1 | YSZ | Ni-SDC | LSM | 900 | CH4-air Rmix = 3.75 | 0.35 | 75 |
| [180,185] | 2007 | Direct-writing | 0.3 | 0.6 | YSZ | Ni-GDC-Pd | LSM-GDC | 900 | CH4-air Rmix = 3.75 | 0.43-0.65 | 12–15 |
| [190] | 2007 | Microfluidic lithography | 0.1 | 0.1 | SDC | Ni-SDC | SSC-SDC | 550 | C3H8-air Rmix = 0.7 | 0.67 | 1.5 |
| [103] | 2007 | Screen printing | 0.3 | 1.2 | CGO | Ni-CGO | SSC | 650 | CH4-air Rmix = 1.5 | 0.75 | 1.5 |
| [103] | 2007 | Micromolding in capillaries | 0.014 | 0.1 | CGO | Ni-CGO | SSC | 650 | CH4-air Rmix = 2 | 0.77 | 17 |
| [149] | 2008 | Direct- writing | 0.517 | 0.13–0.32 | YSZ | Ni-YSZ | LSM | 700 | CH4-air Rmix = 2 | 0.8 | 1.3 |
| [192] | 2009 | Direct- writing | 0.3 | 0.38 | YSZ | Ni-YSZ | LSM-YSZ | 700 | CH4-air Rmix = 2 | 0.73 | 0.7 |
| [151] | 2009 | Direct- writing | 0.114 | 0.26 | YSZ | Ni-YSZ | LSM | 700 | CH4-air Rmix = 2 | 0.8 |
6. Modeling of SC-SOFCs
6.1. Simulation of planar anode-supported SC-SOFCs
6.2. Numerical study of reaction mechanisms in SC-SOFCs
6.3. Efficiency calculations for SC-SOFCs
6.4. Performance modeling of planar electrolyte-supported SC-SOFCs
6.5. Thermodynamic considerations of SC-SOFCs


6.6. Performance studies of SC-SOFCs with coplanar electrodes
7. Applications
7.1. Microsystems and portable power applications
7.2. Energy harvesting applications
7.3. Sensor applications
7.4. System design for practical applications
8. Summary
References and Notes
- Priestnall, M.A.; Kotzeva, V.P.; Fish, D.J.; Nilsson, E.M. Compact mixed-reactant fuel cells. J. Power Sources 2002, 106, 21–30. [Google Scholar] [CrossRef]
- Chen, S.-Z.; Dong, X.-F.; Lin, W.-M. Research progress on single-chamber solid oxide fuel cell. Dianyuan Jishu 2003, 27, 134–136. [Google Scholar]
- Wei, B.; Lu, Z.; Huang, X.Q.; Liu, Z.G.; Al, G.; Su, W.H. Recent advances in single chamber solid oxide fuel cells. Dianyuan Jishu 2006, 30, 243–246. [Google Scholar]
- Buergler, B. E. Single Chamber Solid Oxide Fuel Cells. Ph.D. thesis, ETH, Zurich, Switzerland, 2006. [Google Scholar]
- Yano, M.; Tomita, A.; Sano, M.; Hibino, T. Recent advances in single-chamber solid oxide fuel cells: A review. Solid State Ionics 2007, 177, 3351–3359. [Google Scholar] [CrossRef]
- Hibino, T.; Wang, S.; Kakimoto, S.; Sano, M. Single chamber solid oxide fuel cell constructed from an yttria-stabilized zirconia electrolyte. Electrochem. Solid-State Lett. 1999, 2, 317–319. [Google Scholar] [CrossRef]
- Bay, L.; Horita, T.; Sakai, N.; Ishikawa, M.; Yamaji, K.; Yokokawa, H. Hydrogen solubility in Pr-doped and un-doped YSZ for a one chamber fuel cell. Solid State Ionics 1998, 113-115, 363–367. [Google Scholar] [CrossRef]
- Asano, K.; Hibino, T.; Iwahara, H. SOFC for chemical cogeneration using fuel-air mixed gas. In Proceedings of ECS Transactions - 4th International Symposium on Solid Oxide Fuel Cells, Yokohama, Japan, June, 1995; pp. 58–66.
- Vo, N.X.P.; Yoon, S.P.; Nam, S.W.; Han, J.; Lim, T.H.; Hong, S.A. Fabrication of an anode-supported SOFC with a sol-gel coating method for a mixed-gas fuel cell. Key Eng. Mater. 2005, 277-279, 455–462. [Google Scholar] [CrossRef]
- Raz, S.; Jak, M.J.G.; Schoonman, J.; Riess, I. Supported mixed-gas fuel cells. Solid State Ionics 2002, 149, 335–341. [Google Scholar] [CrossRef]
- Riess, I. The significance of impeded reactions in solid state electrochemistry. Solid State Ionics 2005, 176, 1667–1674. [Google Scholar] [CrossRef]
- Yoon, S.P.; Kim, H.J.; Park, B.T.; Nam, S.W.; Han, J.; Lim, T.H.; Hong, S.A. Mixed-fuels fuel cell running on methane-air mixture. J. Fuel Cell Sci. Technol. 2006, 3, 83–86. [Google Scholar] [CrossRef]
- Shukla, A.K.; Raman, R.K.; Scott, K. Advances in mixed-reactant fuel cells. Fuel Cells 2005, 4, 436–447. [Google Scholar] [CrossRef]
- Riess, I. On the single chamber solid oxide fuel cells. J. Power Sources 2008, 175, 325–337. [Google Scholar] [CrossRef]
- Hibino, T.; Kakimoto, S. Separator Free Single Chamber Solid Electrolyte Fuel Cell and Its Manufacture. Japanese Patent JP2002280015A, 2002. [Google Scholar]
- Goedickemeier, M.; Nussbaum, D.; Kleinlogel, C.; Gauckler, L.J. Solid oxide fuel cells with reaction-selective electrodes. In Proceedings of the 192nd Meeting of the Electrochemical Society, Paris, France, August, 1997; p. 2562.
- Grueneberg, G. Electrochemical conversion of nuclear energy. In Fuel Cells: Modern Processes For The Electrochemical Production Of Energy; Vielstich, W., Ives, D.J.G., Eds.; Wiley-Interscience: New York, USA, 1965; pp. 374–376. [Google Scholar]
- Meng, H.; Wu, M.; Hu, X.X.; Nie, M.; Wei, Z.D.; Shen, P.K. Selective cathode catalysts for mixed-reactant alkaline alcohol fuel cells. Fuel Cells 2006, 6, 447–450. [Google Scholar] [CrossRef]
- Peterson, R.B.; Paul, B.K.; Palmer, T.; Wu, Q.; Jost, W.; Tseng, C.H.T.; Tiwari, S.; Patello, G.; Buck, E.C.; Holladay, J.D.; Shimskey, R.; Humble, P.; MacFarlan, P.; Wainright, J. Radiolytic microscale power generation based on single chamber fuel cell operation. J. Micromech. Microeng. 2007, 17, S250–S256. [Google Scholar] [CrossRef]
- Barton, S.C.; Deng, W.; Gallaway, J.; Levendovsky, S.; Olson, T.S.; Atanassov, P.; Sorkin, M.; Kaufman, A.; Gibbard, H.F. Mixed-feed direct methanol fuel cell: Materials and design solutions. ECS Trans. 2006, 1, 315–322. [Google Scholar]
- Gallaway, J.; Deng, W.; Barton, S.C.; Levendosky, S.; Olson, T.S.; Atanassov, P.; Sorkin, M.; Kaufman, A.; Gibbard, H.F. Mixed-feed direct methanol fuel cell: materials and design solution. In Proceedings of The Electrochemical Society 208th ECS Meeting, Los Angeles, CA, USA, October, 2005; p. 1849.
- Barton, S.C.; Patterson, T.; Wang, E.; Fuller, T.F.; West, A.C. Mixed-reactant, strip-cell direct methanol fuel cells. J. Power Sources 2001, 96, 329–336. [Google Scholar] [CrossRef]
- Scott, K.; Shukla, A.K.; Jackson, C.L.; Meuleman, W.R.A. A mixed-reactants solid-polymer-electrolyte direct methanol fuel cell. J. Power Sources 2004, 126, 67–75. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B. E. Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (Nafion and PTFE) in single chamber microbial fuel cells. Environ. Sci. Technol. 2006, 40, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Haile, S.M.; Ahn, J.; Ronney, P.D.; Zhan, Z.; Barnett, S.A. A thermally self-sustained micro solid-oxide fuel-cell stack with high power density. Nature 2005, 435, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Napporn, T.; Morin, F.; Meunier, M. Evaluation of the actual working temperature of a single-chamber SOFC. Electrochem. Solid-State Lett. 2004, 7, A60–A62. [Google Scholar] [CrossRef]
- Napporn, T.W.; Jacques-Bedard, X.; Morin, F.; Meunier, M. Operating conditions of a single-chamber SOFC. J. Electrochem. Soc. 2004, 151, A2088–A2094. [Google Scholar] [CrossRef]
- Suzuki, T.; Jasinski, P.; Petrovsky, V.; Anderson, H.U.; Dogan, F. Anode supported single chamber solid oxide fuel cell in CH4-air mixture. J. Electrochem. Soc. 2004, 151, A1473–A1476. [Google Scholar] [CrossRef]
- Tuller, H.L. Integration of solid state ionics and electronics: sensors and power sources. J. Ceram. Soc. Jpn. 2004, 112, S1093–S1098. [Google Scholar]
- Bieberle-Huetter, A.; Tuller, H. Fabrication and structural characterization of interdigitated thin film La(1-x)SrxCoO3 (LSCO) electrodes. J. Electroceram. 2006, 16, 151–157. [Google Scholar] [CrossRef]
- Rosenblum, L.; English, R.E. Nuclear-electric systems in space. In Proceedings of Advance Energy Sources and Conversion Techniques, Pasadena, CA, USA, 1958; pp. 243–253.
- Grueneberg, G.; Wicke, W.; Justi, E. Generation of electrical energy. British Patent GB994448, 1961. [Google Scholar]
- Grimes, P.G.; Fiedler, B.; Adam, J. Liquid alkaline fuel cells. In Proceedings of the 15th Power Sources Conference, Session on Primary Fuel Cell Batteries, Fort Monmouth, N.J., USA, 1961; pp. 29–32.
- Eyraud, C.; Lenoir, J.; Gery, M. Fuel cells using electrochemical properties of adsorbents - Piles a combustibles utilisant les proprietes electrochimiques des adsorbats. Acad. Sci. - C. R. 1961, 252, 1599–1600. [Google Scholar]
- van Gool, W. The possible use of surface migration in fuel cells and heterogeneous catalysis. Philips Res. Rep. 1965, 20, 81–93. [Google Scholar]
- Louis, G.A.; Lee, J.M.; Maricle, D.L.; Trocciola, J.C. Solid electrolyte electrochemical cell. US Patent US4248941A, 1981. [Google Scholar]
- Pool, R. Electricity by serendipity. Science 1990, 247, 1034–1035. [Google Scholar] [CrossRef] [PubMed]
- Dyer, C.K. A novel thin-film electrochemical device for energy conversion. Nature 1990, 343, 547–548. [Google Scholar] [CrossRef]
- Dyer, C.K. Primary source of electrical energy using a mixture of fuel and oxidizer. US Patent 4,863,813, 1989. [Google Scholar]
- Taylor, T.M. Efficiency enhancement for solid-electrolyte fuel cell. US Patent US005102750A, 1992. [Google Scholar]
- Dyer, C.K. Compact fuel cell and continuous process for making the cell. US Patent US004988582A, 1991. [Google Scholar]
- Dyer, C.K. Modular fuel cell assembly. US Patent 5,094,928, 1992. [Google Scholar]
- Gottesfeld, S. Thin-film fuel cells. Nature 1990, 345, 673. [Google Scholar] [CrossRef] [PubMed]
- Mallouk, T.E. Miniaturized Electrochemistry. Nature 1990, 343, 515. [Google Scholar] [CrossRef]
- Moseley, P. Sensing reducing gases. Nature 1990, 346, 23. [Google Scholar] [CrossRef]
- Ellgen, P.C. Devices providing electrical energy from fuel/oxygen mixtures. US Patent US005162166A, 1992. [Google Scholar]
- Wang, D.Y.; Kennedy, D.T.; MacAllister, B.W. Method and device for gaseous fuel cell operation. US Patent US005100742A, 1992. [Google Scholar]
- Hibino, T.; Iwahara, H. Simplification of solid oxide fuel cell systems using partial oxidation of methane. Chem. Lett. 1993, 7, 1131–1134. [Google Scholar] [CrossRef]
- Minh, N.Q.; Takahashi, T. Science and Technology of Ceramic Fuel Cells; Elsevier: Amsterdam, Netherlands, 1995. [Google Scholar]
- Buergler, B.E.; Grundy, A.N.; Gauckler, L.J. Thermodynamic equilibrium of single-chamber SOFC relevant methane-air mixtures. J. Electrochem. Soc. 2006, 153, A1378–A1385. [Google Scholar] [CrossRef]
- Kordesch, K.; Simader, G. Fuel Cells and Their Applications; VCH: Weinheim, Germany, 1996. [Google Scholar]
- Hibino, T.; Hashimoto, A.; Inoue, T.; Tokuno, J.I.; Yoshida, S.I.; Sano, M. A low-operating-temperature solid oxide fuel cell in hydrocarbon-air mixtures. Science 2000, 288, 2031–2033. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, P.; Suzuki, T.; Byars, Z.; Dogan, F.; Anderson, H.U. Comparison of anode and electrolyte support configuration of single-chamber SOFC. In Proceedings of ECS Transactions: 8th International Symposium on Solid Oxide Fuel Cells, Paris, France, April/May 2003; pp. 1101–1108.
- Shao, Z.; Kwak, C.; Haile, S.M. Anode-supported thin-film fuel cells operated in a single chamber configuration 2T-I-12. Solid State Ionics 2004, 175, 39–46. [Google Scholar] [CrossRef]
- Hibino, T.; Hashimoto, A.; Inoue, T.; Tokuno, J.I.; Yoshida, S.I.; Sano, M. Single-chamber solid oxide fuel cells at intermediate temperatures with various hydrocarbon-air mixtures. J. Electrochem. Soc. 2000, 147, 2888–2892. [Google Scholar] [CrossRef]
- Yano, M.; Kawai, T.; Okamoto, K.; Nagao, M.; Sano, M.; Tomita, A.; Hibino, T. Single-chamber SOFCs using dimethyl ether and ethanol. J. Electrochem. Soc. 2007, 154, B865–B870. [Google Scholar] [CrossRef]
- Morel, B.; Roberge, R.; Savoie, S.; Napporn, T.; Meunier, M. Catalytic activity and performance of LSM cathode materials in single chamber SOFC. Appl. Catal., A 2007, 323, 181–187. [Google Scholar] [CrossRef]
- Ashcroft, A.T.; Cheetham, A.K.; Foord, J.S.; Green, M.H.; Grey, C.P.; et al. Selective oxidation of methane to synthesis gas using transition metal catalysts. Nature 1990, 344, 319. [Google Scholar] [CrossRef]
- Torniainen, P.M.; Chu, X.; Schmidt, L.D. Comparison of monolith-supported metals for the direct oxidation of methane to syngas. J. Catal. 1994, 146, 1–10. [Google Scholar] [CrossRef]
- Dissanayake, D.; Rosynek, M.P.; Kharas, K.C.C.; Lunsford, J.H. Partial oxidation of methane to carbon monoxide and hydrogen over a Ni/Al2O3 catalyst. J. Catal. 1991, 132, 117–127. [Google Scholar] [CrossRef]
- Peters, K.; Rudolf, M.; Voetter, H. Ueber den Reaktionsverlauf der Methanspaltung. Brennstoff-Chemie 1955, 36, 257–266. [Google Scholar]
- Wheeler, C.; Jhalani, A.; Klein, E.J.; Tummala, S.; Schmidt, L.D. The water-gas-shift reaction at short contact times. J. Catal. 2004, 223, 191–199. [Google Scholar] [CrossRef]
- Tulenin, Y.P.; Sinev, M.Y.; Savkin, V.V.; Korchak, V.N. Dynamic behaviour of Ni-containing catalysts during partial oxidation of methane to synthesis gas. Catal. Today 2004, 91, 155–159. [Google Scholar] [CrossRef]
- Zhang, X.; Hayward, D.O.; Mingos, D.M.P. Further studies on oscillations over nickel wires during the partial oxidation of methane. Catal. Lett. 2003, 86, 235–243. [Google Scholar] [CrossRef]
- Ishihara, T.; Takita, Y. Partial oxidation of methane into syngas with oxygen permeating ceramic membrane reactors. Catal. Surv. Jpn. 2001, 4, 125–133. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Rane, V.H.; Rajput, A.M. Selective oxidation of methane to CO and H2 over unreduced NiO-rare earth oxide catalysts. Catal. Lett. 1993, 22, 289–297. [Google Scholar] [CrossRef]
- Hibino, T.; Hashimoto, A.; Asano, K.; Yano, M.; Suzuki, M.; Sano, M. An intermediate-temperature solid oxide fuel cell providing higher performance with hydrocarbons than with hydrogen. Electrochem. Solid-State Lett. 2002, 5, A242–A244. [Google Scholar] [CrossRef]
- Asano, K.; Hibino, T.; Iwahara, H. A novel solid oxide fuel cell system using the partial oxidation of methane. J. Electrochem. Soc. 1995, 142, 3241–3245. [Google Scholar] [CrossRef]
- Hibino, T.; Wang, S.; Kakimoto, S.; Sano, M. One-chamber solid oxide fuel cell constructed from a YSZ electrolyte with a Ni anode and LSM cathode. Solid State Ionics 2000, 127, 89–98. [Google Scholar] [CrossRef]
- Riess, I.; van der Put, P.J.; Schoonman, J. Solid oxide fuel cells operating on uniform mixtures of fuel and air. Solid State Ionics 1995, 82, 1–4. [Google Scholar] [CrossRef]
- Riess, I. Significance of impeded reactions in solid state electrochemistry-Conspicuous examples. Solid State Ionics 2006, 177, 1591–1596. [Google Scholar] [CrossRef]
- Kellogg, I.D.; Koylu, U.O.; Petrovsky, V.; Dogan, F. Effectiveness of anode in a solid oxide fuel cell with hydrogen/oxygen mixed gases. Int. J. Hydrogen Energy 2009, 34, 5138–5143. [Google Scholar] [CrossRef]
- Akhtar, N.; Decent, S. P.; Loghin, D.; Kendall, K. A three-dimensional numerical model of a single-chamber solid oxide fuel cell. Int. J. Hydrogen Energy 2009, 34, 8645–8663. [Google Scholar] [CrossRef]
- Kellogg, I.D.; Dogan, F.; Petrovsky, V.; Koylu, U.O. Performance of single-chamber SOFC using hydrogen/air mixed-gas fuels. Mater. Sci. Technol. 2006, 1, 371–377. [Google Scholar]
- Kellogg, I.D.; Koylu, U.O.; Petrovsky, V.; Dogan, F. Thermodynamic modeling and testing of the H2/O2/Ni/NiO system. Mater. Sci. Technol. 2007, 2, 1168–1174. [Google Scholar]
- Napporn, T.W.; Savoie, S.; Roberge, R.; Jacques-Bedard, X.; Meunier, M. Single-chamber SOFC: comparing dry and humidified conditions. In Proceedings of the Electrochemical Society– Solid oxide fuel cells IX, Quebec, CA, January, 2005; pp. 371–377.
- Demin, A.K.; Gulbis, F.Y. Efficiency of air-methane mixture fed SOFC. In Proceedings of the 9th CIMTEC-World Forum on New Materials, Symposium VII - Innovative Materials in Advanced Energy Technologies, Florence, Italy, June, 1998; pp. 15–21.
- Kellogg, I.D.; Dogan, F.; Suzuki, T.; Koylu, U.O.; Anderson, H.U.; Petrovsky, V. Single-chamber SOFC operating with hydrocarbon-air and hydrogen-oxygen gas mixtures. ECS Trans. 2007, 7, 971–980. [Google Scholar]
- Zhan, Z.; Liu, J.; Barnett, S.A. Operation of anode-supported solid oxide fuel cells on propane-air fuel mixtures. Appl. Catal., A 2004, 262, 255–259. [Google Scholar] [CrossRef]
- Hibino, T.; Hashimoto, A.; Yano, M.; Suzuki, M.; Yoshida, S.I.; Sano, M. High performance anodes for SOFCs operating in methane-air mixture at reduced temperatures. J. Electrochem. Soc. 2002, 149, A133–A136. [Google Scholar] [CrossRef]
- Cabezas, M.D.; Lamas, D.G.; Bellino, M.G.; Fuentes, R.O.; Walsoe De Reca, N.E.; Larrondo, S.A. Catalytic Behavior of PdONiOSDC composites for partial oxidation of methane: Application as anodes of single-chamber IT-SOFCs. Electrochem. Solid-State Lett. 2009, 12, 34–37. [Google Scholar] [CrossRef]
- Demin, A.K.; Gulbis, F.Y. Zirconia-based SOFC with non-noble electrodes fed by air-methane mixture. Solid State Ionics 2000, 135, 451–456. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, L.; Ran, R.; Shao, Z. Activation of a single-chamber solid oxide fuel cell by a simple catalyst-assisted in-situ process. Electrochem. Commun. 2009, 11, 1563–1566. [Google Scholar]
- Ahn, K.; Kim, H.; Chung, Y.C.; Son, J.W.; Lee, H.W.; Lee, J.H. Catalytic characteristics of perovskite-type oxides under mixed methane and oxygen gases. J. Korean Ceram. Soc. 2008, 45, 232–237. [Google Scholar] [CrossRef]
- Fisher, J.C.; Chuang, S.S.C. Investigating the CH4 reaction pathway on a novel LSCF anode catalyst in the SOFC. Catal. Commun. 2009, 10, 772–776. [Google Scholar] [CrossRef]
- Hartley, A.; Sahibzada, M.; Weston, M.; Metcalfe, I.; Mantzavinos, D. La0.6Sr0.4Co0.2Fe0.8O3 as the anode and cathode for intermediate temperature solid oxide fuel cells. Catal. Today 2000, 55, 197–204. [Google Scholar] [CrossRef]
- Suzuki, T.; Jasinski, P.; Petrovsky, V.; Anderson, H.U.; Dogan, F. Performance of a porous electrolyte in single-chamber SOFCs. J. Electrochem. Soc. 2005, 152, A527–A531. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Z.; Wei, B.; Zhu, R.; Huang, X.; Chen, K.; A, G.; Su, W. Anode-supported micro-SOFC stacks operated under single-chamber conditions. J. Electrochem. Soc. 2007, 154, B588–B592. [Google Scholar] [CrossRef]
- Hao, Y.; Goodwin, D.G. Efficiency and fuel utilization of methane-powered single-chamber solid oxide fuel cells. J. Power Sources 2008, 183, 157–163. [Google Scholar] [CrossRef]
- Kuhn, M. Direct-write microfabrication and characterization of single-chamber micro solid oxide fuel cells with coplanar electrodes. Ph.D. thesis, École Polytechnique de Montréal, Canada, 2009. [Google Scholar]
- Akhtar, N.; Decent, S.P.; Loghin, D.; Kendall, K. Mixed-reactant, micro-tubular solid oxide fuel cells: An experimental study. J. Power Sources 2009, 193, 39–48. [Google Scholar] [CrossRef]
- Morel, B.; Roberge, R.; Savoie, S.; Napporn, T.W.; Meunier, M. An experimental evaluation of the temperature gradient in solid oxide fuel cells. Electrochem. Solid-State Lett. 2007, 10, B31–B33. [Google Scholar] [CrossRef]
- Hibino, T.; Hashimoto, A.; Inoue, T.; Tokuno, J.I.; Yoshida, S.I.; Sano, M. A solid oxide fuel cell using an exothermic reaction as the heat source. J. Electrochem. Soc. 2001, 148, A544–A549. [Google Scholar] [CrossRef]
- Morel, B.; Roberge, R.; Savoie, S.; Napporn, T.W.; Meunier, M. Temperature and performance variations along single chamber solid oxide fuel cells. J. Power Sources 2009, 186, 89–95. [Google Scholar] [CrossRef]
- Akhtar, N.; Decent, S.P.; Kendall, K. Cell Temperature measurements in micro-tubular, single-chamber, solid oxide fuel cells (MT-SC-SOFCs). J. Power Sources 2009. [Google Scholar] [CrossRef]
- Jacques-Bedard, X.; Napporn, T.W.; Roberge, R.; Meunier, M. Performance and ageing of an anode-supported SOFC operated in single-chamber conditions. J. Power Sources 2006, 153, 108–113. [Google Scholar] [CrossRef]
- Stefan, I.C.; Jacobson, C.P.; Visco, S.J.; De Jonghe, L.C. Single chamber fuel cells: flow geometry, rate and composition considerations. Electrochem. Solid-State Lett. 2004, 7, A198–A200. [Google Scholar] [CrossRef]
- Rotureau, D.; Viricelle, J.P.; Pijolat, C.; Caillol, N.; Pijolat, M. Development of a planar SOFC device using screen-printing technology. Journal of the European Ceramic Society 2005, 25, 2633–2636. [Google Scholar] [CrossRef]
- Buergler, B.E.; Siegrist, M.E.; Gauckler, L.J. Single chamber solid oxide fuel cells with mixed ionic electronic conducting electrolyte. In Proceedings of the 5th International Symposium on Ionic and Mixed Conducting Ceramics, Honolulu, Hawaii, USA, Oct. 2004; p. 1748.
- Buergler, B.E.; Siegrist, M.E.; Gauckler, L.J. Single chamber solid oxide fuel cells with integrated current collectors. In Proceedings of the 6th European Solid Oxide Fuel Cell Forum, Lucerne, Switzerland, June, 2004; pp. 1405–1413.
- Buergler, B.E.; Siegrist, M.E.; Gauckler, L.J. Single chamber solid oxide fuel cells with integrated current-collectors. Solid State Ionics 2005, 176, 1717–1722. [Google Scholar] [CrossRef]
- Akhtar, N.; Decent, S.P.; Kendall, K. Structural stability of silver under single-chamber solid oxide fuel cell conditions. Int. J. Hydrogen Energy 2009, 34, 7807–7810. [Google Scholar] [CrossRef]
- Buergler, B.E.; Ochsner, M.; Vuillemin, S.; Gauckler, L.J. From macro- to micro-single chamber solid oxide fuel cells. J. Power Sources 2007, 171, 310–320. [Google Scholar] [CrossRef]
- Kuhn, M.; Napporn, T.W.; Meunier, M.; Therriault, D. Experimental study of current collection in single-chamber micro solid oxide fuel cells with comblike electrodes. J. Electrochem. Soc. 2008, 155, B994–B1000. [Google Scholar] [CrossRef]
- Zabetakis, M.G. Flammability characteristics of combustible gases and vapors. Bulletin 627; U.S. Department of the Interior, Bureau of Mines, 1965. [Google Scholar]
- Crowl, D.A. Understanding explosions; Wiley-AIChE: New York, USA, 2003. [Google Scholar]
- Hibino, T.; Ushiki, K.; Sato, T.; Kuwahara, Y. A novel design for simplifying SOFC system. Solid State Ionics 1995, 81, 1–3. [Google Scholar] [CrossRef]
- Hibino, T.; Asano, K.; Iwahara, H. Solid oxide fuel-cell which can work in uniform-gas phase using partial oxidation of methane. Nippon Kagaku Kaishi 1994, 7, 600–604. [Google Scholar] [CrossRef]
- Hibino, T.; Asano, K.; Iwahara, H. Improvement of Capcius cell using SrCe0.95Yb0.05O3-a as a solid electrolyte. Chem. Lett. 1994, 3, 485–488. [Google Scholar] [CrossRef]
- Hibino, T. Novel SOFC system free from a separator and gas seal. Seramikkusu 1995, 30, 337–340. [Google Scholar]
- Iwahara, H. A one-chamber solid electrolyte fuel cell for chemical cogeneration. Ionics 1998, 4, 409–414. [Google Scholar] [CrossRef]
- Asano, K.; Hibino, T.; Iwahara, H. Studies on solid electrolytes for a new-type SOFC using methane-air mixture. Denki kagaku oyobi kogyo butsuri kagaku 1996, 64, 649–653. [Google Scholar]
- Asano, K.; Iwahara, H. Performance of a one-chamber solid oxide fuel cell with a surface-modified zirconia electrolyte. J. Electrochem. Soc. 1997, 144, 3125–3130. [Google Scholar] [CrossRef]
- Hibino, T.; Kuwahara, Y.; Wang, S. Effect of electrode and electrolyte modification on the performance of one-chamber solid oxide fuel cell. J. Electrochem. Soc. 1999, 146, 2821–2826. [Google Scholar] [CrossRef]
- Wang, S.; Hibino, T. One-chamber SOFC using Ni/LSGM/LSM cell. In Proceedings of the 6th FCDIC Fuel Cell Symposium, Tokyo, Japan, May, 1999; pp. 139–143.
- Hibino, T. Single chamber solid electrolyte fuel cell. Japanese Patent JP2000243412A, 2000. [Google Scholar]
- Hibino, T.; Tsunekawa, H.; Tanimoto, S.; Sano, M. Improvement of a single-chamber solid-oxide fuel cell and evaluation of new cell designs. J. Electrochem. Soc. 2000, 147, 1338–1343. [Google Scholar] [CrossRef]
- Jasinski, P.; Suzuki, T.; Dogan, F.; Anderson, H.U. Impedance spectroscopy of single chamber SOFC. Solid State Ionics 2004, 175, 35–38. [Google Scholar] [CrossRef]
- Lamas, D.G.; Cabezas, M.D.; Fabregas, I.O.; de Reca, N.E.; Lascalea, G.E.; Kodjaian, A.; Vidal, M.A.; Amadeo, N.E.; Larrondo, S.A. NiO/ZrO2-CeO2 anodes for single-chamber solid-oxide fuel cells operating on methane/air mixtures. ECS Trans. 2007, 7, 961–970. [Google Scholar]
- Larrondo, S.A.; Kodjaian, A.; Fabregas, I.; Zimicz, M.G.; Lamas, D.G.; Walsoe de Reca, B.E.; Amadeo, N.E. Methane partial oxidation using Ni/Ce0.9Zr0.1O2 catalysts. Int. J. Hydrogen Energy 2008, 33, 3607–3613. [Google Scholar] [CrossRef]
- Cabezas, M.D.; Lamas, D.G.; Bellino, M.G.; Fuentes, R.O.; de Reca, N.E. Performance of single-chamber intermediate-temperature SOFCs operated in methane/air mixtures using PdO/NiO/CeO2-Sm2O3 anodes. ECS Trans. 2007, 7, 955–960. [Google Scholar]
- Choi, S.H.; Kim, W.S.; Jung, H.Y.; Ahn, S.J.; Lee, J.H.; Lee, H.W.; Kim, J. Structure and conducting properties of porous NiO films for single chamber micro-solid oxide fuel cell. In Proceedings of 207th Meeting of the Electrochemical Society, Quebec City, Canada, May, 2005; p. 812.
- van Rij, L.N.; Le, J.; van Landschoot, R.C.; Schoonman, J. A novel Ni-CERMET electrode based on a proton conducting electrolyte. J. Mater. Sci. 2001, 36, 1069–1076. [Google Scholar] [CrossRef]
- Jou, S.; Wu, T. H. Thin porous Ni-YSZ films as anodes for a solid oxide fuel cell. J. Phys. Chem. Solids 2008, 69, 2804–2812. [Google Scholar] [CrossRef]
- Jasinski, P.; Suzuki, T.; Zhou, X.D.; Dogan, F.; Anderson, H.U. Single chamber solid oxide fuel cell – investigation of cathodes. Ceram. Eng. Sci. Proc. 2003, 24, 293–298. [Google Scholar]
- Suzuki, T.; Jasinski, P.; Dogan, F.; Anderson, H.U. Role of cathode in single chamber SOFC. Ceram. Eng. Sci. Proc. 2003, 24, 257–261. [Google Scholar]
- Dogan, F.; Suzuki, T.; Jasinski, P.; Anderson, H.U. Effect of cathode materials on the performance of single chamber solid oxide fuel cells and module. Ceram. Trans. 2005, 169, 39–47. [Google Scholar]
- Suzuki, T.; Jasinski, P.; Anderson, H.U.; Dogan, F. Role of composite cathodes in single chamber SOFC. J. Electrochem. Soc. 2004, 151, A1678–A1682. [Google Scholar] [CrossRef]
- Hori, M.; Nagasaka, K.; Miyayama, M. Evaluation of electrode performances of single-chamber solid oxide fuel cells. Key Eng. Mater. 2006, 301, 155–158. [Google Scholar] [CrossRef]
- Magnone, E.; Traversa, E.; Miyayama, M. Synthesis and characterization of strontium and iron-doped lanthanum cobaltite nanocrystalline powders for single chamber solid oxide fuel cells. In Proceedings of Electrochemical Society, Solid Oxide Fuel Cells IX: Materials, Quebec City, Canada, May, 2005; pp. 1617–1626.
- Deganello, F.; Esposito, V.; Traversa, E.; Miyayama, M. Electrode performance of nanostructured La1-aSraCo1-bFebO3-x on a Ce0.8Sm0.2O2 electrolyte prepared by citrate nitrate auto-combustion. ECS Trans. 2006, 1, 219–232. [Google Scholar]
- Deganello, F.; Esposito, V.; Miyayama, M.; Traversa, E. Cathode performance of nanostructured La1-aSraCo1-bFebO3-x on a Ce0.8Sm0.2O2 electrolyte prepared by citrate-nitrate autocombustion. J. Electrochem. Soc. 2007, 154, A89–A96. [Google Scholar] [CrossRef]
- Ruiz de Larramendi, I.; Lamas, D.G.; Cabezas, M.D.; Ruiz de Larramendi, J.I.; Walsöe de Reca, N.E.; Rojo, T. Development of electrolyte-supported intermediate-temperature single-chamber SOFCs using Ln0.7Sr0.3Fe0.8Co0.2O3-d (Ln = Pr, La, Gd) cathodes. J. Power Sources 2009, 193, 774–778. [Google Scholar] [CrossRef]
- Lalanne, C. Synthèse et mise en forme de nouveaux matériaux de cathode pour piles ITSOFC: réalisation et tests de cellules. Ph.D. thesis, Université de Bordeaux, France, 2005. [Google Scholar]
- Pinol, S. Stable single-chamber solid oxide fuel cells based on doped ceria electrolytes and La0.5Sr0.5CoO3 as a new cathode. J. Fuel Cell Sci. Technol. 2006, 3, 434–437. [Google Scholar] [CrossRef]
- Morales, M.; Piñol, S.; Segarra, M. Intermediate temperature single-chamber methane fed SOFC based on Gd doped ceria electrolyte and La0.5Sr0.5CoO3-d as cathode. J. Power Sources 2009, 194, 961–966. [Google Scholar] [CrossRef]
- Hibino, T. Single-chamber SOFCs capable of operating in hydrocarbon-air mixtures. Seramikkusu 2001, 36, 486–488. [Google Scholar]
- Asahara, S.; Michiba, D.; Hibino, M.; Yao, T. Single chamber SOFC using BaLaIn2O5.5 solid electrolyte. Electrochem. Solid-State Lett. 2005, 8, 449–451. [Google Scholar] [CrossRef]
- Buergler, B.E.; Santschi, Y.; Felberbaum, M.; Gauckler, L.J. Influence of anode thickness on the electrochemical performance of single chamber solid oxide fuel cells. Ceram. Eng. Sci. Proc. 2006, 27, 37–45. [Google Scholar]
- Morel, B.; Savoie, S.; Roberge, R.; Napporn, T.W.; Meunier, M. Ni-YSZ reduction process under CH4 in a single-chamber SOFC. In Proceedings of the 7th European SOFC Forum, Lucerne, Switzerland, July, 2006.
- Suzuki, T.; Jasinski, P.; Anderson, H.U.; Dogan, F. Single chamber electrolyte supported SOFC module. Electrochem. Solid-State Lett. 2004, 7, A391–A393. [Google Scholar] [CrossRef]
- Yano, M.; Nagao, M.; Okamoto, K.; Tomita, A.; Uchiyama, Y.; Uchiyama, N.; Hibino, T. A single-chamber SOFC stack operating in engine exhaust. Electrochem. Solid-State Lett. 2008, 11, B29–B33. [Google Scholar] [CrossRef]
- Nagao, M.; Yano, M.; Okamoto, K.; Tomita, A.; Uchiyama, Y.; Uchiyama, N.; Hibino, T. A single-chamber SOFC stack: Energy recovery from engine exhaust. Fuel Cells 2008, 8, 322–329. [Google Scholar] [CrossRef]
- Ai, G.; Lu, Z.; Wei, B.; Huang, X.Q.; Chen, K.F.; Su, W.H. Performance of anode-supported single-chamber solid oxide fuel cells. Chin. J. Catal. 2006, 27, 885–889. [Google Scholar]
- Shao, Z.; Mederos, J.; Chueh, W.C.; Haile, S.M. High power-density single-chamber fuel cells operated on methane. J. Power Sources 2006, 162, 589–596. [Google Scholar] [CrossRef]
- Tomita, A.; Hirabayashi, D.; Hibino, T.; Nagao, M.; Sano, M. Single-chamber SOFCs with a Ce0.9Gd0.1O1.95 electrolyte film for low-temperature operation. Electrochem. Solid-State Lett. 2005, 8, A63–A65. [Google Scholar] [CrossRef]
- Hibino, T.; Hashimoto, A.; Suzuki, M.; Yano, M.; Yoshida, S.I.; Sano, M. A solid oxide fuel cell with a novel geometry that eliminates the need for preparing a thin electrolyte film. J. Electrochem. Soc. 2002, 149, A195–A200. [Google Scholar] [CrossRef]
- Jacques-Bedard, X. Conception et caractérisation de piles à électrolyte d′oxyde solide fonctionnant en chambre unique. M.Sc. thesis, École Polytechique de Montréal, Canada, 2005. [Google Scholar]
- Kuhn, M.; Napporn, T.; Meunier, M.; Vengallatore, S.; Therriault, D. Direct-write microfabrication of single-chamber micro solid oxide fuel cells. J. Micromech. Microeng. 2008, 18, 015005–015013. [Google Scholar] [CrossRef]
- Jacques-Bedard, X.; Napporn, T.W.; Roberge, R.; Meunier, M. Coplanar electrodes design for a single-chamber SOFC: Assessment of the operating parameters. J. Electrochem. Soc. 2007, 154, B305–B309. [Google Scholar] [CrossRef]
- Kuhn, M.; Napporn, T.W.; Meunier, M.; Vengallatore, S.; Therriault, D. Miniaturization limits for single-chamber micro solid oxide fuel cells with coplanar electrodes. J. Power Sources 2009, 194, 941–949. [Google Scholar] [CrossRef]
- Pinol, S.; Segarra, M.; Capdevila, X. Preparation and electrical properties of intermediate temperature one chamber solid oxide fuel cells based on ceria electrolytes. In Proceedings of the 1st European Fuel Cell Technology and Applications Conference, Rome, Italy, December, 2005; p. 201.
- Pinol, S.; Morales, M.; Espiell, F. Low temperature anode-supported solid oxide fuel cells based on gadolinium doped ceria electrolytes. J. Power Sources 2007, 169, 2–8. [Google Scholar] [CrossRef]
- Shao, Z.; Haile, S.M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 2004, 431, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zheng, Y.; Lin, Y.; Ran, R.; Shao, Z.; Farrusseng, D. A comparative study of La0.8Sr0.2MnO3 and La0.8Sr0.2Sc0.1Mn0.9O3 as cathode materials of single-chamber SOFCs operating on a methane-air mixture. J. Power Sources 2009, 191, 225–232. [Google Scholar] [CrossRef]
- Wei, B.; Lu, Z.; Huang, X.; Liu, M.; Chen, K.; Su, W. Enhanced performance of a single-chamber solid oxide fuel cell with an SDC-impregnated cathode. J. Power Sources 2007, 167, 58–63. [Google Scholar] [CrossRef]
- Liu, M.; Lü, Z.; Wei, B.; Huang, X.; Chen, K.; Su, W. Study on impedance spectra of La0.7Sr0.3MnO3 and Sm0.2Ce0.8O1.9-impregnated La0.7Sr0.3MnO3 cathode in single chamber fuel cell condition. Electrochim. Acta 2009, 54, 4726–4730. [Google Scholar] [CrossRef]
- Haile, S.M.; Shao, Z. Barium strontium cobalt iron oxide based perovskite mixed conducting materials as cathode materials for intermediate temperature solid oxide fuel cells both in dual chamber and single chamber configuration. Patent WO2005001958-A2, 2005. [Google Scholar]
- Ahn, J.; Ronney, P.D.; Shao, Z.; Haile, S.M. A thermally self-sustaining miniature solid oxide fuel cell. In Proceedings of the 5th International Energy Conversion Engineering Conference, St. Louis, MI, USA, June, 2007.
- Ahn, J.; Shao, Z.; Ronney, P.D.; Haile, S.M. A thermally self-sustaining miniature solid oxide fuel cell. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Seattle, WA, USA, November, 2007; pp. 117–122.
- Hao, Y.; Shao, Z.; Mederos, J.; Lai, W.; Goodwin, D.G.; Haile, S.M. Recent advances in single-chamber fuel-cells: Experiment and modeling. Solid State Ionics 2006, 177, 2013–2021. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, Y.; Ran, R.; Shao, Z.; Jin, W.; Xu, N.; Ahn, J. Initialization of a methane-fueled single-chamber solid-oxide fuel cell with NiO + SDC anode and BSCF + SDC cathode. J. Power Sources 2008, 179, 640–648. [Google Scholar] [CrossRef]
- Tomita, A.; Teranishi, S.; Nagao, M.; Hibino, T.; Sano, M. Comparative performance of anode-supported SOFCs using a thin Ce0.9Gd0.1O1.95 electrolyte with an incorporated BaCe0.8Y0.2O3-a layer in hydrogen and methane. J. Electrochem. Soc. 2006, 153, A956–A960. [Google Scholar] [CrossRef]
- Ahn, J.; Ronney, P.D.; Shao, Z.; Haile, S.M. A thermally self-sustaining miniature solid oxide fuel cell. J. Fuel Cell Sci. Technol. 2009, 6, 041004–041007. [Google Scholar] [CrossRef]
- Song, H.S.; Min, J.H.; Kim, J.; Moon, J. Phase stability of Sm0.5Sr0.5CoO3 cathodes for on-planar type, single-chamber, solid oxide fuel cells. J. Power Sources 2009, 191, 269–274. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Z.; Wei, B.; Huang, X.; Chen, K.; Su, W. Effect of the cell distance on the cathode in single chamber SOFC short stack. J. Electrochem. Soc. 2009, 156, B1253–B1256. [Google Scholar] [CrossRef]
- Wei, B.; Lü, Z.; Huang, X.; Liu, M.; Jia, D.; Su, W. A novel design of single-chamber SOFC micro-stack operated in methane-oxygen mixture. Electrochem. Commun. 2009, 11, 347–350. [Google Scholar] [CrossRef]
- Hertz, J.; Tuller, H.L. Single chamber solid oxide fuel cell comprises planar electrolyte layer; planar selectively active cathode contacting electrolyte layer and planar selectively active anode contacting electrolyte layer, all layers having specific thickness. Patent WO2007024907-A2, 2007. [Google Scholar]
- Kamijo, M.; Rouveyre, L. Solid oxide fuel cell for electric power generation, has several air holes that are located in electrolyte such that mixed gas of fuel gas circulates in air holes. Japanese Patent JP2007242429-A, 2007. [Google Scholar]
- Anderson, H.; Suzuki, T.; Jasinski, P.; Petrovsky, V. Electrochemical energy extraction from hydrocarbon fluids to convert potential energy to electricity comprises positioning anode and cathode layers on porous, non-densified yttria-doped zirconia electrolyte substrate. US Patent US2008038592-A1, 2008. [Google Scholar]
- Nagata, A.; Kimura, T. Selective control of voltage polarity in a single-chamber solid-oxide fuel cell using the same catalytic electrodes with different sizes. IEEJ Trans. Electr. Electron. Eng. 2008, 3, 569–573. [Google Scholar] [CrossRef]
- Kim, H.; Choi, S.H.; Kim, W.S.; Lee, J.H.; Lee, H.W.; Kim, J. Development of single chamber solid oxide fuel cells with coplanar micro-electrode array. In Proceedings of the 207th Meeting of the Electrochemical Society, Quebec, Canada, May, 2005; p. 1213.
- Fleig, J.; Tuller, H.L.; Maier, J. Electrodes and electrolytes in micro-SOFCs: A discussion of geometrical constraints. Solid State Ionics 2004, 174, 261–270. [Google Scholar] [CrossRef]
- Hibino, T.; Ushiki, K.; Kuwahara, Y. New concept for simplifying SOFC system. Solid State Ionics 1996, 91, 69–74. [Google Scholar] [CrossRef]
- Tomita, A.; Namekata, Y.; Nagao, M.; Hibino, T. Room-temperature hydrogen sensors based on an In3+-Doped SnP2O7 Proton Conductor. J. Electrochem. Soc. 2007, 154, J172–J176. [Google Scholar] [CrossRef]
- Yoshikata, K.; Sakamoto, H. Base material for solid oxide fuel cell, has several polygonal or circular shaped fuel elctrodes and air elecrodes, arranged on surface of electrolyte. Japanese Patent JP2006004672-A, 2006. [Google Scholar]
- Kuhn, M.; Napporn, T.; Meunier, M.; Therriault, D.; Vengallatore, S. Fabrication and testing of coplanar single-chamber micro solid oxide fuel cells with geometrically complex electrodes. J. Power Sources 2008, 177, 148–153. [Google Scholar] [CrossRef]
- Kearl, D.A.; Peterson, R.B. Single chamber solid oxide fuel cell architecture for high temperature operation. US Patent US2003190505, 2003. [Google Scholar]
- Kearl, D.A.; Peterson, R.B. Single chamber solid oxide fuel cell architecture for high temperature operation. US Patent US20050238946A1, 2005. [Google Scholar]
- Ahn, S.J.; Kim, Y.B.; Moon, J.; Lee, J.H.; Kim, J. Influence of patterned electrode geometry on performance of co-planar type single chamber SOFC. J. Power Sources 2007, 171, 511–516. [Google Scholar] [CrossRef]
- Chung, C.Y.; Chung, Y. C. Computational modeling of the performance characteristics of micro scale single-chamber IT-SOFC system. In Proceedings of Electrochemical Society - SOFC IX, Quebec City, Canada, May, 2005; pp. 670–678.
- Chung, C.Y.; Chung, Y.C.; Kim, J.; Lee, J.; Lee, H.W. Numerical modeling of micro single-chamber ceria-based SOFC. J. Electroceram. 2006, 17, 959–964. [Google Scholar] [CrossRef]
- Chung, C.Y.; Chung, Y.C. Performance characteristics of micro single-chamber solid oxide fuel cell: Computational analysis. J. Power Sources 2006, 154, 35–41. [Google Scholar] [CrossRef]
- Son, J.W.; Ahn, S.J.; Kim, S.M.; Kim, H.; Choi, S.H.; Moon, J.; Kim, H.R.; Kim, S.E.; Lee, J.H.; Lee, H.W.; Kim, J. Fabrication and operation of co-planar type single chamber solid oxide fuel cells. In Proceedings of the 7th European SOFC Forum, Lucerne, Switzerland, July, 2006.
- Ahn, S.J.; Kim, Y.B.; Moon, J.; Lee, J.H.; Kim, J. Co-planar type single chamber solid oxide fuel cell with micro-patterned electrodes. J. Electroceram. 2006, 17, 689–693. [Google Scholar] [CrossRef]
- Crumlin, E.J.; La O, G.J.; Shao-Horn, Y. Architectures and performance of high-voltage, microscale single-chamber solid oxide fuel cell stacks. ECS Trans. 2007, 7, 981–986. [Google Scholar]
- Lee, H.W.; Kim, E.K.; Son, J.W.; Kim, H.C.; Kim, H.R.; Kim, J.S.; Lee, J.H.; Song, H.S. Single-chamber solid oxide fuel cell having partition interposed between two homogeneous or heterogeneous electrodes and method for manufacturing the same. Korean Patent KR724120-B1, 2008. [Google Scholar]
- Kim, H.C.; Lee, H.W.; Kim, S.M.; Song, H.S.; Lee, J.H.; Kim, J.S.; Son, J.W.; Ahn, S.J. Single-chamber solid oxide fuel cell using partition formed on one surface of electrolyte and interposed between two electrodes. Korean Patent KR724119-B1, 2008. [Google Scholar]
- Ahn, S.J.; Lee, J.H.; Kim, J.; Moon, J. Single-chamber solid oxide fuel cell with micropatterned interdigitated electrodes. Electrochem. Solid-State Lett. 2006, 9, A228–A231. [Google Scholar] [CrossRef]
- Min, J.H.; Ahn, S.J.; Moon, J.; Kim, J.; Lee, H.W. Single-chamber mini-solid oxide fuel cells operated at a lower temperature. ECS Trans. 2007, 7, 947–953. [Google Scholar]
- Jasinski, P. Micro solid oxide fuel cells and their fabrication methods. Microelectron. Int. 2008, 25, 42–48. [Google Scholar] [CrossRef]
- Kuhn, M.; Napporn, T.W.; Meunier, M.; Therriault, D. Single-chamber micro solid oxide fuel cells: Study of anode and cathode materials in coplanar electrode design. Solid State Ionics 2009. submitted. [Google Scholar]
- Yang, C.C. T.; Wei, W.C. J.; Roosen, A. Reaction kinetics and mechanisms between La0.65Sr0.3MnO3 and 8 mol% yttria-stabilized zirconia. J. Am. Ceram. Soc. 2004, 87, 1110–1116. [Google Scholar] [CrossRef]
- Nagata, A.; Nosaka, H.; Yoshimura, Y.; Kimura, T. Room temperature operation of surface-conduction single chamber fuel cell using a novel inorganic electrolyte as proton conductor. IEEJ Trans. Electr. Electron. Eng. 2008, 3, 593–595. [Google Scholar] [CrossRef]
- Choi, S.H.; Hwang, C.S.; Lee, H.W.; Kim, J. Fabrication of Gd2O3-doped CeO2 thin films for single-chamber-type solid oxide fuel cells and their characterization. J. Electrochem. Soc. 2009, 156, B381–B385. [Google Scholar] [CrossRef]
- Coia, C. Fabrication et caracterisation d′une micro-pile a combustible a electrolyte oxyde solide. M.Sc. thesis, École Polytechnique de Montréal, Canada, 2002. [Google Scholar]
- Bieberle-Hutter, A.; Sogaard, M.; Tuller, H. L. Electrical and electrochemical characterization of microstructured thin film La1-xSrxCoO3 electrodes. Solid State Ionics 2006, 177, 1969–1975. [Google Scholar] [CrossRef]
- Viricelle, J. P.; Pijolat, C.; Riviere, B.; Rotureau, D.; Briand, D.; de Rooij, N. F. Compatibility of screen-printing technology with micro-hotplate for gas sensor and solid oxide micro fuel cell development. Sens. Actuators, B 2006, 118, 263–268. [Google Scholar] [CrossRef]
- Ahn, S.J.; Moon, J. Vacuum-assisted microfluidic lithography of ceramic microstructures. J. Am. Ceram. Soc. 2005, 88, 1171–1174. [Google Scholar] [CrossRef]
- Moon, J.H.; Ahn, S.J. Micro-patterning method, micro-pattern substrate, and single chamber solid oxide fuel cell manufactured thereby. Korean Patent KR2006038649-A, 2006. [Google Scholar]
- Ahn, S.J.; Lee, J.H.; Kim, J.; Moon, J. Single-chamber solid oxide fuel cell with micropatterned interdigitated electrodes. In Proceedings of Electrochemical Society - SOFC IX, Quebec City, Canada, May, 2005; pp. 378–383.
- Moon, J.H.; Kim, Y.B.; Kim, J.S. Preparation method of electrochemical cell by preparing paste and substrate, and patterning the paste on the substrate by robo-dispensing, and solid oxide fuel cell prepared by the method. Korean Patent KR2007015731-A, 2007. [Google Scholar]
- Kim, H.C.; Park, J.K.; Lee, H.W.; Lee, J.H.; Kim, J.S.; Son, J.W.; Choi, S.H. Single chamber solid oxide fuel cell with isolated electrolyte. Patent WO2007073015-A1, 2007. [Google Scholar]
- Kuhn, M.; Napporn, T.; Meunier, M.; Therriault, D.; Vengallatore, S. Direct-write microfabrication of single-chamber solid oxide fuel cells with interdigitated electrodes. Mater. Res. Soc. Symp. Proc. 2007, 972, 211–216. [Google Scholar]
- Kuhn, M.; Rao, B.R.; Therriault, D. Viscoelastic inks for direct-write microfabrication of single-chamber micro solid oxide fuel cells with coplanar thick electrodes. Mater. Res. Soc. Symp. Proc. 2009, 1179, 111–116. [Google Scholar] [CrossRef]
- Kim, Y.B.; Ahn, S.J.; Moon, J.; Kim, J.; Lee, H.W. Direct-write fabrication of integrated planar solid oxide fuel cells. J. Electroceram. 2006, 17, 683–687. [Google Scholar] [CrossRef]
- Yoshikata, K.; Sakamoto, H. Solid-acid compound type fuel cell has interconnectors arranged in through-holes formed in electrolyte, for connecting air electrodes and fuel electrodes arranged on both surfaces of electrolyte. Japanese Patent JP2005310501-A, 2005. [Google Scholar]
- Yoshikata, K.; Sakamoto, H. Single chambered solid oxide fuel cell has interconnector used for attaching air electrode and fuel electrode so that adjacent single cell are connected in series. Japanese Patent JP2006221884-A, 2005. [Google Scholar]
- Lu, Z.; Su, W.; Liu, J.; Huang, X.; Liu, Z.; Miao, J.; Li, C. Battery composed of single-chamber solid oxide fuel cells (SOFCs). Chinese Patent CN1564361, 2005. [Google Scholar]
- Hibino, T.; Ushiki, K.; Kuwabara, Y. Non-diaphragm solid electrolyte type fuel cell for co-generation. Japanese Patent JP8264195, 1996. [Google Scholar]
- Zhu, B.; Meng, G.; Mellander, B.E. Non-conventional fuel cell systems: new concepts and development. J. Power Sources 1999, 79, 30–36. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A. Fuel cell systems explained; John Wiley & Sons: Chichester, UK, 2000. [Google Scholar]
- Koh, Y.H.; Sun, J.J.; Choi, W.Y.; Kim, H.E. Design and fabrication of three-dimensional solid oxide fuel cells. J. Power Sources 2006, 161, 1023–1029. [Google Scholar] [CrossRef]
- Hao, Y.; Pantano, C.; Goodwin, D.G. A single-chamber solid oxide fuel cell model with detailed chemistry. In Proceedings of the Fourth Joint Meeting of the U.S. Sections of the Combustion Institute: Western States, Central States, Eastern States, Philadelphia, PA, United States, March 2005; pp. B39/31–B39/36.
- Hao, Y.; Pantano, C.; Goodwin, D.G. A two-dimensional model of a single-chamber SOFC with hydrocarbon fuels. In Proceedings of Electrochemical Society - SOFC IX, Quebec City, Canada, May, 2005; pp. 771–779.
- Hao, Y.; Goodwin, D.G. Numerical modeling of single-chamber SOFCs with hydrocarbon fuels. J. Electrochem. Soc. 2007, 154, B207–B217. [Google Scholar] [CrossRef]
- Akhtar, N.; Decent, S.P.; Loghin, D.; Kendall, K. An isothermal numerical model of single-chamber solid oxide fuel cells. In Proceedings of the 8th European SOFC Forum, Lucerne, Switzerland, 2008. B0715, Abstract 003.
- Hao, Y.; Goodwin, D.G. Numerical study of heterogeneous reactions in an SOFC anode with oxygen addition. J. Electrochem. Soc. 2008, 155, B666–B674. [Google Scholar] [CrossRef]
- Hao, Y.; Goodwin, D.G. Numerical study of heterogeneous reactions in an SOFC anode with oxygen addition. ECS Trans. 2007, 7, 1859–1867. [Google Scholar]
- Shiratori, Y.; Yamazaki, Y. A novel high-voltage single-chamber SOFC with series connected cells. I. Operating characteristics of two-segments cell. In Proceedings of Electrochemical Society - SOFC VII, Lucerne, Switzerland, July, 2006; pp. 1012–1021.
- Shiratori, Y.; Yamazaki, Y. Study of high voltage single chamber SOFC with series connected cells I. IV-IP characteristics of two segments cell. Electrochem. 2001, 69, 92–97. [Google Scholar]
- Uchiyama, N. Single chamber type solid oxide fuel cell for motor vehicle, has solid oxide fuel cell unit accommodated inside exhaust-gas flow path of internal combustion engine. Patent WO2007094262-A1, 2007. [Google Scholar]
- Evans, J. Anon Fuel cells that don′t feel the cold. Chem. World 2005, 2, 20. [Google Scholar]
- Nikbin, D. Micro SOFCs: why small is beautiful. Fuel Cell Rev. 2006, 3, 21–24. [Google Scholar]
- Horiuchi, M.; Tokutake, Y.; Suganuma, S.; Yoshiike, J.; Katagiri, F. Solid oxide fuel cell. US Patent US2008187806A1, 2007. [Google Scholar]
- Weingaertner, D.; Kalika, V.; Wiser, C. Fuel cell system e.g. solid oxide fuel cell (SOFC) system has venturi with apertures for radially injecting fluid for mixing with air to deliver air-fuel mixture to catalytic reactor. US Patent US2008187794-A1, 2008. [Google Scholar]
- Lazaroff, D.; Mardilovich, P.; Champion, D.; Herman, G.S. Fuel cell manufacture e.g. for solid oxide fuel cell used in e.g. computer, involves performing chemical mechanical planarization to remove anode, electrolyte and cathode layers formed on specific topographic pattern. US Patent US20040086754-A1, 2004. [Google Scholar]
- Kawai, H. Single chambered fuel cell for use in e.g. motor vehicle, has main electrode layer with recesses in which sub-electrode layers whose height is identical with respect to height of convex surface of main electrode layer, are arranged. Japanese Patent JP2009087711-A, 2009. [Google Scholar]
- Scheifers, S.M.; Klosterman, D.H.; Chason, M.K.; Wyatt, K.W. Portable fuel cell device including a water trap. US Patent US005723229A, 1998. [Google Scholar]
- Yoshikata, K.; Sakamoto, H.; Kotani, K. Solid oxide fuel cell e.g. single-room type solid oxide fuel cell, has gas passageway that connects specific surface of board and fuel electrode formed on surface of board. Japanese Patent JP2007323957-A, 2007. [Google Scholar]
- Yoshikata, K.; Sakamoto, H. Single chamber type solid oxide shape fuel cell. Japanese Patent JP2006139960, 2006. [Google Scholar]
- Kotani, K.; Yoshikata, K.; Sakamoto, H. Single-chamber solid oxide fuel cell has interconnector with hydrogen separation layer at fuel electrode side of unit cell. Japanese Patent JP2007273428-A, 2007. [Google Scholar]
- Yoshikata, K.; Sakamoto, H.; Mikami, G. Single chamber type solid oxide fuel cell electric power generation system mixes fuel gas containing methane, and oxygen to used mixed gas ejected from electric power generator and circulates mixed gas to electric power generator. Japanese Patent JP2006114368-A, 2006. [Google Scholar]
- Yoshikata, K.; Sakamoto, H.; Mikami, G. Single chamber-type solid oxide fuel cell electric power generation system has single chamber-type solid oxide fuel cell to eject circulation gas containing both fuel gas and oxidizing agent gas. Japanese Patent JP2006114373-A, 2006. [Google Scholar]
- Yoshikata, K.; Kotani, K. Single-chambered solid oxide fuel cell has electrolyte extended downward along periphery of fuel electrode and air electrode of two different single cells respectively and connected to upper surface of porous substrate. Japanese Patent JP2008041540-A, 2008. [Google Scholar]
- Kotani, K.; Yoshikata, K. Single-chambered solid oxide fuel cell for generating electric power, has single cells and electro-conductive porous substrate(s) inserted between adjoining single cells, and single cell comprises fuel electrode and air electrode. Japanese Patent JP2008060001-A, 2008. [Google Scholar]
- Kotani, K.; Yoshikata, K. Single chamber solid oxide fuel cell has single cell consisting of air electrode as outermost layer and another single cell consisting of fuel electrode as outermost layer at both ends of porous block. Japanese Patent JP2008059869-A, 2008. [Google Scholar]
- Yoshikata, K.; Kotani, K. Single chambered solid oxide fuel cell has dense substance substrate, single cell having fuel electrode, electrolyte and air electrode provided on electrolyte formed on fuel electrode, and porous substrate on air electrode. Japanese Patent JP2008077887-A, 2008. [Google Scholar]
- Yoshikata, K.; Kotani, K. Single-chambered solid oxide fuel cell for electric power generation, has substrate and single cell(s) having air electrode formed on electrolyte formed on fuel electrode and communicating hole, formed on substrate. Japanese Patent JP2008047380-A, 2008. [Google Scholar]
- Yoshikata, K.; Sakamoto, H.; Kotani, K. Jig for stack structure of single chamber solid oxide fuel cell, has pair of guides arranged at both sides of base and moving mechanism, so that moving mechanism is contacted/separated with respect to base. Japanese Patent JP2008034235-A, 2008. [Google Scholar]
- Yoshikata, K.; Kotani, K. Jig for stack of single-chambered solid oxide fuel cell, has lower-side mesh element that extends in outer peripheral surface of supporting element which contacts fuel electrode of fuel cell. Japanese Patent JP2008084551-A, 2008. [Google Scholar]
- Kotani, K.; Yoshikata, K. Stack structure of single chambered solid oxide fuel cell has buffer layer with cushioning properties, provided in side wall surface of recess opposite to fuel electrode and air electrode in single cell. Japanese Patent JP2008078069-A, 2008. [Google Scholar]
- Kotani, K.; Yoshikata, K. Single chamber solid oxide fuel cell for electric power generation, has fuel electrode arranged in downstream side so as to allow gas to flow through air electrode arranged in upstream side. Japanese Patent JP2008084745-A, 2008. [Google Scholar]
- Yoshikata, K.; Kotani, K. Inter-connector of single chambered solid oxide fuel cell, consists of rectangular block which is formed with set of through holes that are connected together in specific order by gas flow channels. Japanese Patent JP2008226557-A, 2008. [Google Scholar]
- Kotani, K.; Yoshikata, K. Solid oxide fuel cell has insulating electrolyte that covers fuel electrode and inner wall face of through-hole in porous substrate. Japanese Patent JP2008251241-A, 2008. [Google Scholar]
- Yoshikata, K.; Kotani, K. Solid oxide fuel cell e.g. single room type fuel cell, for use as battery, has modified layer of porous positioning in through-hole, which has partial oxidation activity and supplied gas contacting fuel electrode through through-hole. Japanese Patent JP2008257885-A, 2008. [Google Scholar]
- Yoshikata, K.; Kotani, K. Solid oxide fuel cell comprises electrolyte, fuel electrode which is arranged on electrolyte, air electrode which is spaced apart with fuel electrode and is arranged on electrolyte and modified layer which has partial oxidation activity. Japanese Patent JP2008257886-A, 2008. [Google Scholar]
- Kotani, K.; Yoshikata, K. Single-chamber solid oxide fuel cell system has air electrode with left opening connected with supply pipe for supplying mixed gas to air electrode and right opening facing discharge pipe for discharging mixed gas from container. Japanese Patent JP2008251277-A, 2008. [Google Scholar]
- Kotani, K.; Yoshikata, K. Single chamber solid oxide fuel cell for electric power generation, has vertical flow passages that are extended from horizontal gas flow passage in electrolyte between fuel electrode and air electrode. Japanese Patent JP2008251384-A, 2008. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kuhn, M.; Napporn, T.W. Single-Chamber Solid Oxide Fuel Cell Technology—From Its Origins to Today’s State of the Art. Energies 2010, 3, 57-134. https://doi.org/10.3390/en3010057
Kuhn M, Napporn TW. Single-Chamber Solid Oxide Fuel Cell Technology—From Its Origins to Today’s State of the Art. Energies. 2010; 3(1):57-134. https://doi.org/10.3390/en3010057
Chicago/Turabian StyleKuhn, Melanie, and Teko W. Napporn. 2010. "Single-Chamber Solid Oxide Fuel Cell Technology—From Its Origins to Today’s State of the Art" Energies 3, no. 1: 57-134. https://doi.org/10.3390/en3010057
APA StyleKuhn, M., & Napporn, T. W. (2010). Single-Chamber Solid Oxide Fuel Cell Technology—From Its Origins to Today’s State of the Art. Energies, 3(1), 57-134. https://doi.org/10.3390/en3010057