Long Term Performance Study of a Direct Methanol Fuel Cell Fed with Alcohol Blends
Abstract
:1. Introduction
2. Experimental
2.1. Electrodes

2.2. MEA Preparation
2.3. Experimental Procedure
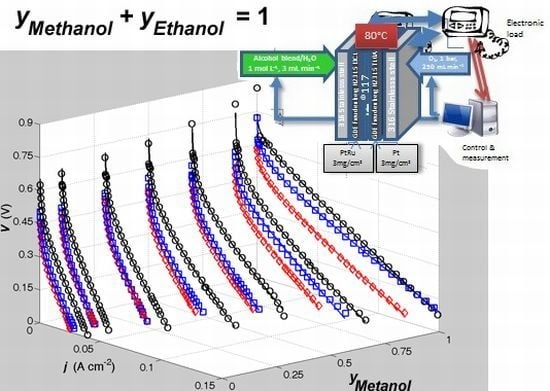
- A series of polarization curves have been recorded for a custom DMFC fuelled by liquid MeOH/EtOH blends in water at a constant total alcohol concentration c of 1 mol L−1. The alcohol blend composition was gradually varied from 1 mol L−1 MeOH to 1 mol L−1 EtOH using intermediate MeOH/EtOH molar compositions (yM and yE, respectively) of 0.90/0.10, 0.70/0.30, 0.50/0.50, 0.30/0.70 and 0.10/0.90. The oxidant was always pure oxygen. The fuel cell temperature was kept constant at 80 °C in every case. Before each test, the cell was preconditioned three times. Polarization curves were recorded three consecutive times for each different fuel composition.
- The fuel cell was filled with 1 mol L−1 aqueous MeOH solution for one week, and then the polarization curve of the fuel cell fed with 1 mol L−1 aqueous MeOH was recorded. This step was repeated until the polarization curve remained unchanged.
| Parameter | Value |
|---|---|
| Total alcohol (MeOH/EtOH) concentration (mol L−1) | 1.0 |
| Alcohol volumetric flow rate (mL min−1) | 3.0 |
| Oxygen volumetric flow rate (mL min−1) | 250 |
| Oxygen pressure (bar) | 1.0 |
| Temperature (°C) | 80 |
3. Polarization Curve Model for Mixed Alcohol Fuel
3.1. Curve Model
- ●
- The global anode reaction order, (chemical kinetics);
- ●
- The anode charge transfer coefficient (electrode charge transfer);
- ●
- The cell resistance by area (global charge transport).
3.2. Mixture Model
| Parameter | MeOH | EtOH |
|---|---|---|
| Standard Nernst potential E° (V) | 1.214 | 1.146 |
| Number of electrons considered in the anodic reaction za | 6 | 12 |
| Membrane thickness lm (m) | 1.78 × 10−5 [25] | 1.78 × 10−5 [25] |
| Backing layer thickness (anode and cathode) lb (m) | 3.00 × 10−5 [25] | 3.00 × 10−5 [25] |
| Catalyst layer thickness (anode and cathode) lc (m) | 2.0×10−6 [26] | 2.0 × 10−6 [26] |
| Cathode transfer αc coefficient | 1 [22,26] | 1 [22,26] |
| Electro-osmotic drag coefficient nd | 3.16 [26] | 3.16 [26] |
| Diffusion coeff. of oxygen in the cathode backing layer (m2 s−1) | 3.38 × 10−5 [27] | 3.38 × 10−5 [27] |
| Order of reaction (cathode) γc | 1 [22,26] | 1 [22,26] |
| Anode reference exchange current density multiplied by the specific surface area (A cm−3) | [22] | [26] |
| Cathode exchange current density j0,c (A cm−2) | 1.87 × 10−8 [21] | 1.87 × 10−8 [21] |
| Diffusion coeff. of alcohol in the anode backing layer (m2 s−1) | 2.984 × 10−9 [28] | 2.984 × 10−9 [28] |
| Diffusion coeff. of alcohol in the membrane Dm (m2 s−1) | 2.148 × 10−9 [20] | 2.97 × 10−9 [27] |
4. Results
4.1. Experimental Performance of a DMFC Fed with MeOH/EtOH Aqueous Solution Mixtures

4.2. Response of the Fuel Cell to Aqueous MeOH after Being Operated with MeOH/EtOH Mixtures. Fuel Cell Recovery Process

4.3. Curve Fitting

5. Conclusions
- ●
- Fuel cell performance declines as the ethanol content in MeOH/EtOH mixture increases.
- ●
- The fuel cell recovery process after operation with MeOH/EtOH mixtures only partly reverts the loss of fuel cell performance.
- ●
- The anodic global reaction order reaches a value that is independent of the fuel composition (almost recovering its original value) as the fuel cell operation time increases.
- ●
- The global charge transport of the fuel cell decreases linearly with the ethanol content in the fuel blend, but is not time-dependent as its original value is restored after each recovery process.
- ●
- The anode charge transfer coefficient shows progressive decay with the ethanol proportion in each series, and does not return to its original value after the recovery processes. This points to the fact that the electrode charge transfer must be a very important cause of the long term loss of fuel cell performance.
Nomenclature
| c | Global alcohol concentration |
| E | Nernst potential under operating conditions |
| EtOH | Ethanol |
| j | Current density |
| MeOH | Methanol |
| P | Fixed parameters required by the model |
| Rint | Global resistance times area |
| V | Output voltage of the fuel cell |
| yE | Molar proportion of ethanol in the alcohol mixture |
| yM | Molar proportion of methanol in the alcohol mixture |
Subscripts
| a | Anode, anodic |
| c | Cathode, cathodic |
| E | Ethanol |
| M | Methanol |
Greek Letters
| α | Charge transfer coefficient |
| γ | Reaction order |
Overpotential | |
Activation overpotential | |
Concentration overpotential | |
Ohmic overpotential |
Acknowledgements
References
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Nishimura, A.; Shibuya, K.; Morimoto, A.; Tanaka, S.; Hirota, M.; Nakamura, Y.; Kojima, M.; Narita, M.; Hu, E. Dominant factor and mechanism of coupling phenomena in single cell of polymer electrolyte fuel cell. Appl. Energy 2012, 90, 73–79. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Gallorini, F.; Ottaviano, A. Dynamic analysis of PEMFC-based CHP systems for domestic application. Appl. Energy 2012, 91, 13–28. [Google Scholar] [CrossRef]
- Ahn, S.H.; Choi, I.; Kwon, O.J.; Kim, J.J. One-step co-electrodeposition of Pt–Ru electrocatalysts on carbon paper for direct methanol fuel cell. Chem. Eng. J. 2012, 181–182, 276–280. [Google Scholar] [CrossRef]
- Léon, A. Hydrogen Technology: Mobile and Portable Applications; Springer-Verlag Berlin-Heidelberg: Berlin, Germany, 2008; pp. 11–150. [Google Scholar]
- Rand, D.A.J.; Dell, R.M. Hydrogen Energy, Challenges and Prospects; RSC Publishing: Cambridge, UK, 2008; pp. 28–33. [Google Scholar]
- Hotza, D.; Diniz da Costa, J.C. Fuel cells development and hydrogen production from renewable resources in Brazil. Int. J. Hydrog. Energy 2008, 33, 4915–4935. [Google Scholar] [CrossRef]
- Gnansounou, E. Production and use of lignocellulosic bioethanol in Europe: Current situation and perspectives. Bioresour. Technol. 2010, 101, 4842–4850. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, G.; Stergiopoulos, V.; Song, S.; Tsiakaras, P. Direct ethanol fuel cells: The effect of the cell discharge current on the products distribution. Appl. Catal. B 2010, 10, 157–164. [Google Scholar] [CrossRef]
- Zhou, W.J.; Zhou, B.; Li, W.Z.; Zhou, Z.H.; Song, S.Q.; Sun, G.Q.; Xin, Q.; Douvartzides, S.; Goula, M.; Tsiakaras, P. Performance comparison of low-temperature direct alcohol fuel cells with different anode catalysts. J. Power Sources 2004, 126, 16–22. [Google Scholar] [CrossRef]
- Song, S.; Tsiakaras, P. Recent progress in direct alcohol proton exchange membrane fuel cells (DE-PEMFCs). Appl. Catal. B Environ. 2006, 63, 187–193. [Google Scholar] [CrossRef]
- Antolini, E. Catalysts for direct ethanol fuel cells. J. Power Sources 2007, 170, 1–12. [Google Scholar] [CrossRef]
- Leo, T.J.; Raso, M.A.; Navarro, E.; Sánchez de la Blanca, E.; Villanueva, M.; Moreno, B. Response of a direct methanol fuel cell to fuel change. Int. J. Hydrog. Energy 2010, 35, 11642–11648. [Google Scholar] [CrossRef]
- Wongyao, N.; Therdthianwong, A.; Therdthianwong, S. Performance direct alcohol fuel cells fed with mixed methanol/ethanol solutions. Energy Convers. Manag. 2011, 52, 2676–2681. [Google Scholar] [CrossRef]
- Kulikovsky, A.A. Analytical Modelling of Fuel Cells, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Leo, T.J.; Raso, M.A.; Navarro, E.; Sánchez de la Blanca, E. Comparative exergy analysis of direct alcohol fuel cells using fuel mixtures. J. Power Sources 2011, 196, 1178–1183. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 87th ed.; Taylor and Francis: Boca Raton, FL, USA, 2007; pp. 8–84. [Google Scholar]
- Pramanik, H.; Basu, S. Modeling and experimental validation of overpotentials of a direct ethanol fuel cell. Chem. Eng. Process. Process Intensif. 2010, 49, 635–642. [Google Scholar] [CrossRef]
- Kulikovsky, A.A. A method for analysis of DMFC performance curves. Electrochem. Commun. 2003, 5, 1030–1036. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Miao, Z.; Liu, Y. Two-phase modelling of mass transfer characteristics of a direct methanol fuel cell. Appl. Therm. Eng. 2009, 29, 1998–2008. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Srinivasan, S.; Appleby, A.J.; Martin, C.R. Temperature dependence of the electrode kinetics of oxygen reduction at the Pt-Nafion® interface—A microelectrode investigation. J. Electrochem. Soc. 1992, 139, 2530–2537. [Google Scholar] [CrossRef]
- Xu, C.; Faghri, A. Water transport characteristics in a passive liquid-feed DMFC. Int. J. Heat Mass Tran. 2010, 53, 1951–1966. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, T.S.; Yang, W.W. Modeling of water transport through the membrane electrode assembly for direct methanol fuel cells. J. Power Sources 2008, 178, 291–308. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.S.; Yang, W.W.; Xu, C. Two-dimensional two-phase thermal model for passive direct methanol fuel cell. J. Power Sources 2008, 175, 276–287. [Google Scholar] [CrossRef]
- Kulikovsky, A.A. The voltage-current curve of a direct methanol fuel cell: “Exact” and fitting equations. Electrochem. Commun. 2002, 4, 939–946. [Google Scholar] [CrossRef]
- Andreadis, G.M.; Podias, A.K.M.; Tsiakaras, P.E. A model-based parametric analysis of a direct ethanol polymer electrolyte membrane fuel cell performance. J. Power Sources 2009, 194, 397–407. [Google Scholar] [CrossRef]
- Andreadis, G.M.; Podias, A.K.M.; Tsiakaras, P.E. The effect of the parasitic current on the direct ethanol polymer electrolyte membrane fuel cell operation. J. Power Sources 2008, 181, 214–227. [Google Scholar] [CrossRef]
- Arisetty, S.; Advani, S.G.; Prasad, A.K. Methanol diffusion rates through the anode diffusion layer in DMFC from limiting current measurements. Heat Mass Tran. 2008, 44, 1199–1206. [Google Scholar] [CrossRef]
- Leo, T.J.; Raso, M.A.; Navarro, E.; Mora, E. Perspectives on the Design and Use of Direct Alcohol Fuel Cells Fed by Alcohol Blends. In Proceedings of Communication to 6th Dubrovnik Conference on Sustainable Development of Energy Water and Environment Systems, Dubrovnik, Croatia, 25–29 September 2011.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leo, T.J.; Raso, M.A.; Navarro, E.; Mora, E. Long Term Performance Study of a Direct Methanol Fuel Cell Fed with Alcohol Blends. Energies 2013, 6, 282-293. https://doi.org/10.3390/en6010282
Leo TJ, Raso MA, Navarro E, Mora E. Long Term Performance Study of a Direct Methanol Fuel Cell Fed with Alcohol Blends. Energies. 2013; 6(1):282-293. https://doi.org/10.3390/en6010282
Chicago/Turabian StyleLeo, Teresa J., Miguel A. Raso, Emilio Navarro, and Eleuterio Mora. 2013. "Long Term Performance Study of a Direct Methanol Fuel Cell Fed with Alcohol Blends" Energies 6, no. 1: 282-293. https://doi.org/10.3390/en6010282
APA StyleLeo, T. J., Raso, M. A., Navarro, E., & Mora, E. (2013). Long Term Performance Study of a Direct Methanol Fuel Cell Fed with Alcohol Blends. Energies, 6(1), 282-293. https://doi.org/10.3390/en6010282