Preparation of Polybenzimidazole-Based Membranes and Their Potential Applications in the Fuel Cell System
Abstract
: Various polybenzimidazole (PBI)-based ion-exchange films were prepared and thoroughly characterized by Fourier transform infrared (FT-IR) spectroscopy, proton conductivity, and water uptake for possible use as fuel cell membranes. Upon the increase in the flexibility of the PBI-based polymer films (e.g., poly(oxyphenylene benzimidazole) (OPBI) and sulfonated OPBI (s-OPBI)), the membranes exhibited slightly improved proton conductivity, but significantly increased dimensional changes. To reduce the dimensional changes (i.e., increase the stability), the cross-linking of the polymer films (e.g., cross-linked OPBI (c-OPBI) and sulfonated c-OPBI (sc-OPBI)) was accomplished using phosphoric acid. Interestingly, the sc-OPBI membrane possessed a greatly increased proton conductivity (0.082 S/cm), which is comparable to that of the commercially available Nafion membrane (0.09 S/cm), while still maintaining slightly better properties regarding the dimensional change and water uptake than those of the Nafion membrane.1. Introduction
The proton exchange membrane fuel cell (PEMFC), which possesses the ability to withstand high operating temperatures and is energy efficient, is a promising device for resolving both energy and environmental concerns [1,2]. The development of proton conductive membranes for PEMFC applications that are inexpensive and highly stable is challenging. Currently, Nafion (Dupont's fluorinated electrolyte membrane) is the most widely-used fuel cell membrane; however, it has some drawbacks such as a high price, low ion conductivity at long operation times, and decreased performance at high temperatures [3]. As such, most research has focused on the preparation and modification of various proton conductive membranes that are inexpensive and provide better performance and properties [4–7].
Acid doping into aromatic polymer materials is one of the more interesting approaches in designing proton conductive films with good thermal and chemical stabilities for alternative fuel cell membranes (e.g., polyimides, polysulfones, polybenzoxazoles, poly(ether ether ketones), and polybenzimidazoles (PBIs)) [2,4–6,8–13]. In this study, we have employed PBI, which is very cheap and exhibits thermally and chemically stable properties under various conditions [6,14–17]. PBI has a heterocyclic benzimidazole ring with an amphoteric character, which can be easily modified with strong acids (such as phosphoric acid and/or sulfuric acid) to be used as an ionic conductive membrane [3,18–20]. Unfortunately, PBI films are very brittle and rigid, often resulting in low proton conductivity. Thus, we attempted to control the flexibility of the PBI material by employing dicarboxydiphenyl ether (DCDPE) (typically isophthalic acid (IPA) for PBI synthesis). The resulting poly(oxyphenylene benzimidazole) (OPBI) possesses an oxygen in the PBI backbone that can increase the flexibility of the film [21]. Subsequently, the OPBI membrane is further sulfonated by sulfuric acid and/or cross-linked by phosphoric acid to improve the proton conductivity and maintain a good stability at high operating temperatures, which could surpass the limitations of current proton conductive membranes. The main focus of our study in this stage is to examine the synthesis and characterization of various flexible PBI-based membranes for potential PEMFC applications.
2. Experiment
2.1. Materials
Diaminobenzene (DABz), IPA, polyphosphoric acid (PPA), N,N-dimethylacetamide (DMAc), sulfuric acid, ethanol, 4,4′-DCDPE, Eaton's reagent, dimethyl sulfoxide (DMSO), and phosphoric acid were purchased from Aldrich (St. Louis, MO, USA) and used without any purification. A glass plate (20 cm × 28 cm) was used to prepare various polymer membranes.
2.2. Preparation of PBI, s-PBI, o-PBI, s-OPBI, and sc-OPBI Membranes
2.2.1. Preparation of PBI [22]
DABz (3.115 g) and IPA (4.018 g) were mixed with PPA (60 g) and placed in a round-bottom flask equipped with a reflux condenser with an inlet for nitrogen. The mixture was heated to 190 °C for 20 h. The polymerized PBI powder was collected and then dissolved in DMAc to prepare 10 wt% of the PBI solution. Incompletely dissolved PBI powder in DMac was removed by a simple centrifugation step. The resulting polymer solution was subsequently cast on the glass plate to form a ∼20 µm thin film (i.e., the PBI membrane). The cast film was dried at 80 °C for 24 h and immersed in a water bath. The final OPBI membrane was heated again at 60 °C for 24 h to remove any residual solvents (Scheme 1).
2.2.2. Preparation of Sulfonated PBI (s-PBI)
To improve ion conductivity, the PBI film was treated with 98 wt% sulfuric acid at room temperature for three days (sulfonated-PBI, s-PBI membrane). This acid-treated PBI film was washed with ethanol and subsequently dried at 60 °C for 24 h (Scheme 2).
2.2.3. Preparation of OPBI [21,23]
The s-PBI membrane exhibited extremely low proton conductivity due to its highly rigid structure; we attempted to provide flexibility to the s-PBI membrane by replacing IPA with DCDPE repeating units in the polymer backbone (Scheme 3). The structure of DCDPE appears to be the extended form of IPA, with an oxygen and a phenyl group that provide flexibility to the entire polymer backbone (OPBI structure). Specifically, OPBI was prepared by using DCDPE (0.51646 g), DABz (0.41644 g) (10:9.7 ratio) and Eaton's reagent (30 mL) at 140 °C for 2 h under nitrogen gas [16]. The collected OPBI was then dissolved in DMSO (3 wt%) and then cast on the glass plate. The rest of treatment was similar to the preparation of the PBI membrane.
2.2.4. Sulfonation of the OPBI Film (s-OPBI)
To improve ion conductivity, the OPBI film was systematically treated with various concentrations of sulfuric acid (0–70 wt%) for 24 h (Scheme 4). The acid treated s-OPBI membranes were washed with ethanol and subsequently dried at 60 °C for 24 h. The OPBI-based membranes exhibited a high dimensional change in the water that could cause stability issues at high operating temperatures.
2.2.5. Cross-Linking and Sulfonation of the OPBI Films (sc-OPBI)
As the s-OPBI film has shown a low stability and high dimensional change in water, we attempted to prepare cross-linked and sulfonated OPBI films that could introduce the enhanced stability and reduced dimensional change (Scheme 5). In our study, the cross-linking of the OPBI membrane was initially achieved by immersing the membrane in a phosphoric acid (85 wt%) solution. The cross-linking reaction was completed at 80 °C in an oven for 2 h and then at 180 °C for 20 h. The resulting c-OPBI membranes were washed with hot water (∼80 °C) a few times and dried at 80 °C in an oven overnight. It is important to note that phosphoric acid is employed as the cross-linking agent and also works as an acid-doping material in the film which may increase the proton conductivity. Subsequently, the sulfonation of these c-OPBI films was accomplished similarly via the sulfonation of OPBI films using 98 wt% sulfuric acid.
2.3. Characterization of s-PBI, s-OPBI and sc-OPBI Membranes
Fourier transform infrared (FT-IR) spectroscopy measurements (Jasco FT-IR 620, Jasco Corporation, Easton, MD, USA, resolution 4.0, average of 100 scans) were carried out in order to confirm the synthesis of various PBI-based membranes (e.g., s-PBI, s-OPBI, and sc-OPBI).
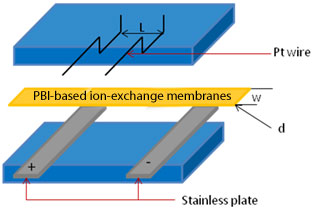
For the proton conductivity measurement, all membrane samples were soaked in 1 M HCl for 4 h, allowing for complete protonation of the sulfonate group. The proton conductivity was then measured with the impedance analyzer (WEIS 500, Hitachi, Japan). The complex impedance in the range of 10 Hz to 1 MHz was monitored using a stainless steel electrode. Figure 1 shows the scheme of the proton conductivity measurement system, where the stainless plates were the electrode and Pt wires were the voltage sensing probes. The direct current resistance of the membranes was obtained when the Z′ phase was at 0. The proton conductivity was then calculated based on the following Equation (1) [24]:
Measurements of the water uptake and dimensional change of the membranes in the 80 °C water were also performed to evaluate the stability of the membranes. Five membrane samples (50 mm × 50 mm) were prepared and immersed in 80 °C water for 24 h. After the water uptake, the residual water from the sample's surface was removed by the absorption paper. The water uptake and dimensional change of the membranes were then recorded and calculated using the following Equations (2) and (3):
where Wwet = weight of a fully water-absorbed membrane; and Wdry = weight of a dry membrane.
where Awet = area of a fully water-absorbed membrane; and Adry = area of a dry membrane.
3. Results
3.1. FT-IR of PBI and s-PBI Membranes
Figure 2 shows the infrared (IR) spectra of PBI and s-PBI with characteristic peaks at 3500–2500 cm−1 for the N–H bond and at 1630 cm−1 for the C=N bond, respectively. The distinctive in-plane deformation peak of the imidazole ring was also observed at 1460 cm−1 [25]. Upon sulfonation, the s-PBI membrane clearly showed a new broad peak at 1053 cm−1, corresponding to the symmetric stretch of the sulfonate group (SO3−) [22,26].
3.2. FT-IR of OPBI, s-OPBI and sc-OPBI Membranes
As the successful sulfonation of the PBI film was examined by FT-IR, we analyzed the OPBI film and the series of cross-linked OPBI (c-OPBI)-based films as a function of the degree of sulfonation. Figure 3 shows the IR spectra patterns of OPBI, c-OPBI, and various sc-OPBI membranes. All OPBI-based films exhibited a peak at 1250 cm−1 for the C–O bond, indicating the successful incorporation of DCDPE into the polymer backbone. The cross-linked films with phosphoric acid (c-OPBI and sc-OPBI) were also confirmed by the presence of peaks at 3214 cm−1 and at 1423 cm−1 for O–H and N–O peaks, respectively. The peak at 1084 cm−1 for all sc-OPBI films clearly indicates successful sulfonation [3,18,22,26].
3.3. Proton Conductivity of s-PBI, s-OPBI and sc-OPBI Membranes
Proton conductivity (where protons can freely move around in the ion exchange membrane via the hopping mechanism [27]) is one of the most important characteristics of ion exchange membranes when evaluating fuel cell performance [14,28]. As such, the proton conductivity of the prepared films was compared to that of commercially available Nafion, a sulfonated tetrafluoroethylene-based membrane. We observed extremely low proton conductivity of the s-PBI membrane (Figure 4) compared to that of the Nafion membrane. Although we speculated that the sulfonate groups in our PBI-based films could play an important role in the movement of protons, the rigidity of the polymer backbone and overall polymer structure (e.g., the polymer backbone and side chains) resulted in the low proton conductivity. Upon the formation of a highly flexible OPBI film and its sulfonated OPBI derivatives (s-OPBI), these films have shown notably increased proton conductivity. Based on our systematic studies, the proton conductivity generally increased as the concentration of sulfuric acid increased (up to 70%; the highest proton conductivity from OPBI occurred with 70% sulfuric acid).
In addition, the proton conductivity of the OPBI membrane with the treatment of 70% sulfuric acid was measured as a function of the sulfonation time (Figure 5). The optimized incubation time required for the best proton conductivity was found to be the 24-h treatment of the OPBI membrane with 70% sulfuric acid at 80 °C (the prolonged incubation time appeared to degrade the film, leading to inconsistent results). The s-OPBI membrane notably increased the proton conductivity, but was still far below that of the Nafion membrane.
In a separate study, we evaluated the stability, water uptake, and dimensional change of s-OPBI (Figure 6). The proton conductivity of the s-OPBI membrane relatively increased as the water uptake increased (i.e., a 24-h sulfonated membrane), indicating a possible relationship between these two properties [19,29]. More thorough studies are under way to fully understand this relationship. It is important note that the s-OPBI film has a much better water uptake ability than that of the Nafion membrane; the s-OPBI film could be utilized for PEMFC applications under low humidity conditions. Our s-PBI membrane with a high water uptake/transport property could maintain a consistent fuel cell performance in a low humidity environment.
Too much dimensional change in the membranes in water is one of the major problems for fuel cell performance and long term stability. It was reported that the performance of Nafion in a fuel cell system is dramatically decreased due to the high dimensional change that occurs under high temperature and moisture conditions [30]. Although the s-OPBI membranes generally showed a higher water uptake property, these membranes overall exhibited a lower dimensional change than that of Nafion. The s-OPBI membrane with the highest proton conductivity (24 h of sulfonation) showed a similar dimensional change to that of Nafion. This observation led us to modify s-OPBI with a cross-linking agent to prepare sc-OPBI (sulfonated and c-OPBI) membranes to reduce the dimensional change.
It has been reported that PBI films can increase both the thermal stability and proton conductivity upon treatment with aqueous phosphoric acid (i.e., acid doping); we initially attempted to prepare c-OPBI membranes by treating them with phosphoric acid. Surprisingly, the proton conductivity of this bare c-OPBI without sulfonation was greatly enhanced (0.035 S/cm at 80 °C) simply due to the presence of the cross-links with phosphoric acid. This enhancement implies that the OH group of phosphoric acid in the c-OPBI membrane plays an important role for the transportation of protons. The proton conductivity of sc-OPBI was then examined as a function of the initial sulfuric acid concentration (Figure 7). In addition, when the c-OPBI membranes were systematically treated with varying concentrations of sulfuric acid (increased from 0 wt% to 70 wt%), the proton conductivity gradually increased, presumably because of more sulfonation. The sc-OPBI membrane with 70 wt% sulfuric acid showed comparable proton conductivity to Nafion (0.09 S/cm at 80 °C). This sc-OPBI membrane with highly increased proton conductivity could be used as an alternative hydrocarbon membrane for PEMFC applications.
Figure 8 shows the water uptake and dimensional change of the sc-OPBI membranes. When the concentration of sulfuric acid increased, both the water uptake property and the dimensional change gradually increased. While the increase of the water uptake (40%) by the sc-OPBI membrane with 70 wt% sulfuric acid was greater than that of Nafion (32%), the dimensional change of the same membrane (11.8%) was much smaller than that of Nafion (30.6%). This observation indicates that the cross-linking process with phosphoric acid not only improves the proton conductivity but also reduces the dimensional change (which is related to the long-term stability).
4. Conclusions
In this study, the preparation and characterization of various PBI-based films for possible PEMFC applications were accomplished. The presence of sulfonate groups in various PBI-based films was successfully confirmed by FT-IR. The s-OPBI membrane with an oxygen atom in the PBI backbone had slightly improved proton conductivity over that of s-PBI due to its increased flexibility. In addition, the s-OPBI film exhibited a higher water uptake with a moderate dimensional change. Upon the cross-linking of s-OPBI by phosphoric acid, the resulting sc-OPBI interestingly exhibited the highly enhanced proton conductivity of 0.081 S/cm, which is comparable to the conductivity of the commercially available Nafion (0.09 S/cm). Furthermore, the sc-OPBI membrane possessed a slightly higher water uptake (40%) than that of Nafion (32%), but the dimensional change of the film was found to be much lower (11.5%) than that of Nafion (30.6%). Since the sc-OPBI membrane has a high proton transfer capability and low dimensional change, it has the potential to be used as a highly effective and practical membrane in the field of PEMFC systems.
Acknowledgments
This study was supported by the R&D Fund of the Ministry of Knowledge Economy (No. 10038355).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sopian, K.; Wan Daud, W.R. Challenges and future developments in proton exchange membrane fuel cells. Renew. Energy 2006, 31, 719–727. [Google Scholar]
- Zhang, H.; Shen, P.K. Recent development of polymer electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar]
- Jouanneau, J.; Mercier, R.; Gonon, L.; Gebel, G. Synthesis of sulfonated polybenzimidazoles from functionalized monomers: Preparation of ionic conducting membranes. Macromolecules 2007, 40, 983–990. [Google Scholar]
- Hwang, K.; Kwon, B.; Byun, H. Preparation of PVdF nanofiber membranes by electrospinning and their use as secondary battery separators. J. Membr. Sci. 2011, 378, 111–116. [Google Scholar]
- Lufrano, F.; Baglio, V.; Staiti, P.; Aricò, A.S.; Antonucci, V. Development and characterization of sulfonated polysulfone membranes for direct methanol fuel cells. Desalination 2006, 199, 283–285. [Google Scholar]
- Staiti, P.; Lufrano, F.; Aricò, A.S.; Passalacqua, E.; Antonucci, V. Sulfonated polybenzimidazole membranes—Preparation and physico-chemical characterization. J. Membr. Sci. 2001, 188, 71–78. [Google Scholar]
- Zaidi, S.M.J.; Mikhailenko, S.D.; Robertson, G.P.; Guiver, M.D.; Kaliaguine, S.J. Proton conducting composite membranes from polyether ether ketone and heteropolyacids for fuel cell applications. J. Membr. Sci. 2000, 173, 17–34. [Google Scholar]
- Haufe, S.; Stimming, U. Proton conducting membranes based on electrolyte filled microporous matrices. J. Membr. Sci. 2001, 185, 95–103. [Google Scholar]
- Sirk, A.H.C.; Hill, J.M.; Kung, S.K.Y.; Birss, V.I. Effect of redox state of PtRu electrocatalysts on methanol oxidation activity. J. Phys. Chem. B 2004, 108, 689–695. [Google Scholar]
- Gao, Y.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Kaliaguine, S. Synthesis of poly(arylene ether ether ketone ketone) copolymers containing pendant sulfonic acid groups bonded to naphthalene as proton exchange membrane materials. Macromolecules 2004, 37, 6748–6754. [Google Scholar]
- Lufrano, F.; Gatto, I.; Staiti, P.; Antonucci, V.; Passalacqua, E. Sulfonated polysulfone ionomer membranes for fuel cells. Solid State Ion. 2001, 145, 47–51. [Google Scholar]
- Watari, T.; Fang, J.; Tanaka, K.; Kita, H.; Okamoto, K.-I.; Hirano, T. Synthesis, water stability and proton conductivity of novel sulfonated polyimides from 4,4′-bis(4-aminophenoxy)biphenyl-3,3′-disulfonic acid. J. Membr. Sci. 2004, 230, 111–120. [Google Scholar]
- Xing, P.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Kaliaguine, S. Sulfonated poly(aryl ether ketone)s containing the hexafluoroisopropylidene diphenyl moiety prepared by direct copolymerization, as proton exchange membranes for fuel cell application. Macromolecules 2004, 37, 7960–7967. [Google Scholar]
- Mader, J.; Xiao, L.; Schmidt, T.J.; Benicewicz, B.C. Polybenzimidazole/acid complexes as high-temperature membranes. Adv. Polym. Sci. 2008, 216, 63–124. [Google Scholar]
- Mader, J.A.; Benicewicz, B.C. Sulfonated polybenzimidazoles for high temperature PEM fuel cells. Macromolecules 2010, 43, 6706–6715. [Google Scholar]
- Ueda, M.; Sato, M.; Mochizuki, A. Poly(benzimidazole) synthesis by direct reaction of diacids and diamines. Macromolecules 1985, 18, 2723–2726. [Google Scholar]
- Xiao, L.; Zhang, H.; Jana, T.; Scanlon, E.; Chen, R.; Choe, E.-W.; Ramanathan, L.S.; Yu, S.; Benicewicz, B.C. Synthesis and characterization of pyridine-based polybenzimidazoles for high temperature polymer electrolyte membrane fuel cell applications. Fuel Cells 2005, 5, 287–295. [Google Scholar]
- Glipa, X.; Bonnet, B.; Mula, B.; Jones, D.J.; Rozière, J. Investigation of the conduction properties of phosphoric and sulfuric acid doped polybenzimidazole. J. Mater. Chem. 1999, 9, 3045–3049. [Google Scholar]
- Li, Y.S.; Zhao, T.S.; Yang, W.W. Measurements of water uptake and transport properties in anion-exchange membranes. Int. J. Hydrog. Energy 2010, 35, 5656–5665. [Google Scholar]
- Vogel, H.; Marvel, C.S. Polybenzimidazoles, new thermally stable polymers. J. Polym. Sci. 1961, 50, 511–539. [Google Scholar]
- Kim, T.H.; Kim, S.K.; Lim, T.W.; Lee, J.C. Synthesis and properties of poly(aryl ether benzimidazole) copolymers for high-temperature fuel cell membranes. J. Membr. Sci. 2008, 323, 362–370. [Google Scholar]
- Deimede, V.; Voyiatzis, G.A.; Kallitsis, J.K.; Qingfeng, L.; Bjerrum, N.J. Miscibility behavior of polybenzimidazole/sulfonated polysulfone blends for use in fuel cell applications. Macromolecules 2000, 33, 7609–7617. [Google Scholar]
- Liu, Y.; Shi, Z.; Xu, H.; Fang, J.; Ma, X.; Jin, Y. Preparation, characterization, and properties of novel polyhedral oligomeric silsesquioxane–Polybenzimidazole nanocomposites by Friedel–Crafts reaction. Macromolecules 2010, 43, 6731–6738. [Google Scholar]
- Han, A.; Park, S.-J.; Shin, J.-S.; Kim, S. Preparation and electrochemical behaviors of polymer electrolyte based on PEO/PMMA containing Li ion. Korean Chem. Eng. Res. 2009, 47, 476–480. [Google Scholar]
- Álvarez-Gallego, Y.; Ruffmann, B.; Silva, V.; Silva, H.; Lozano, A.E.; de la Campa, J.G.; Nunes, S.P.; de Abajo, J. Sulfonated polynaphthalimides with benzimidazole pendant groups. Polymer 2008, 49, 3875–3883. [Google Scholar]
- Xu, H.; Chen, K.; Guo, X.; Fang, J.; Yin, J. Synthesis of novel sulfonated polybenzimidazole and preparation of cross-linked membranes for fuel cell application. Polymer 2007, 48, 5556–5564. [Google Scholar]
- Sone, Y.; Ekdunge, P.; Simonsson, D. Proton conductivity of Nafion 117 as measured by a four-electrode AC impedance method. J. Electrochem. Soc. 1996, 143, 1254–1259. [Google Scholar]
- Kim, D.S.; Robertson, G.P.; Guiver, M.D. Comb-shaped poly(arylene ether sulfone)s as proton exchange membranes. Macromolecules 2008, 41, 2126–2134. [Google Scholar]
- Ge, S.; Li, X.; Yi, B.; Hsing, I.M. Absorption, desorption, and transport of water in polymer electrolyte membranes for fuel cells. J. Electrochem. Soc. 2005, 152, 1149–1157. [Google Scholar]
- Shang, M.; Matsuyama, H.; Teramoto, M.; Lloyd, D.R.; Kubot, N. Preparation and membrane performance of poly(ethylene-co-vinyl alcohol) hollow fiber membrane via thermally induced phase separation. Polymer 2003, 44, 7441–7447. [Google Scholar]













© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hwang, K.; Kim, J.-H.; Kim, S.-Y.; Byun, H. Preparation of Polybenzimidazole-Based Membranes and Their Potential Applications in the Fuel Cell System. Energies 2014, 7, 1721-1732. https://doi.org/10.3390/en7031721
Hwang K, Kim J-H, Kim S-Y, Byun H. Preparation of Polybenzimidazole-Based Membranes and Their Potential Applications in the Fuel Cell System. Energies. 2014; 7(3):1721-1732. https://doi.org/10.3390/en7031721
Chicago/Turabian StyleHwang, Kyungho, Jun-Hyun Kim, Sung-Yul Kim, and Hongsik Byun. 2014. "Preparation of Polybenzimidazole-Based Membranes and Their Potential Applications in the Fuel Cell System" Energies 7, no. 3: 1721-1732. https://doi.org/10.3390/en7031721
APA StyleHwang, K., Kim, J. -H., Kim, S. -Y., & Byun, H. (2014). Preparation of Polybenzimidazole-Based Membranes and Their Potential Applications in the Fuel Cell System. Energies, 7(3), 1721-1732. https://doi.org/10.3390/en7031721