1. Introduction
Photovoltaic properties and carrier transport of perovskite based solar cells have been studied for improving charge separation of electrons and holes in a light absorbing layer and carrier transport [
1,
2,
3,
4,
5,
6,
7]. Organic-inorganic hybrid heterojunction solar cells containing the CH
3NH
3PbI
3 perovskite compound have the great advantage of fabricating on mesoporous TiO
2 as the electronic transporting layer and 2,2′,7,7′-tetrakis-(
N,N-di-4-methoxyphenylamino)-9,9′-spirobifluorene (spiro-OMeTAD) as the hole-transporting layer (HTL) [
8,
9,
10]. The effects of halogen doping and HTL on photovoltaic properties and microstructure on organic-inorganic hybrid heterojunction solar cells using the perovskite compound have been studied for improving photovoltaic performance and optical properties. In particular, the influence of halogen doping on iodide, bromine and chlorine halide perovskite structures in the hybrid solar cells have been characterized for optimizing band gap, crystal structure, optical and photovoltaic properties and conversion efficiency [
11,
12,
13]. The role of halogen doping using iodine (I), bromine (Br) and chlorine (Cl) compounds as the dopant and the HTL on the photovoltaic and structural properties is an important factor to optimize the improvement of carrier-generation, carrier diffusion, transporting properties and photovoltaic performance. Quantitative investigation into the photovoltaic and optical properties of the perovskite structure with molar ratio dependence of I, Cl and Br compounds has been performed [
13]. Control of halogen doping with molar ratio between I, Cl and Br in perovskite crystal structure will provide the best condition for the photovoltaic performance, the crystal structure, carrier mobility and electronic structure with band gaps between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) and the optical properties with a wide range of absorption. The insertion of both Cl and Br into the perovskite structure promoted the carrier-generation, and expanded carrier diffusion and lifetime, along with reducing the charge recombination in the active layers for improving the photovoltaic performance [
14,
15,
16,
17,
18,
19,
20]. A detailed description of the photovoltaic properties, structural analysis, and theoretical investigation using X-ray diffraction pattern (XRD), X-ray photoelectron spectroscopy, scanning electronic microscopy (SEM) and density-functional theory calculation of halogen-doped perovskite layer of CH
3NH
3PbI
3−xCl
x, CH
3NH
3PbBr
3−xCl
x, CH
3NH
3SnX
3 (X = Cl, Br, I), [HC(NH
2)
2]
0.83Cs
0.17Pb(I
0.6Br
0.4)
3], antimony-doped CH
3NH
3PbI
3, CH
3NH
3Pb
1−xGe
xI
3, CH
3NH
3Pb
1−xTl
xI
3 and CH
3NH
3Pb
1−xIn
xI
3 on TiO
2 layers has been reported [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29].
The purpose of this study is to investigate influence of halogen doping at variable molar ratio of I, Cl, and Br in inorganic-organic hybrid solar cells using CH3NH3PbI3−x−yBrxCly perovskite compounds. The influence of a small amount of halogen doping of I, Cl and Br compounds in the perovskite structure on the photovoltaic and optical properties and charge transport behavior will be investigated for improving the electronic transporting behavior and photovoltaic properties. By using XRD, SEM and energy dispersive X-ray spectroscopy (EDX), microstructure and surface morphologies of the perovskite layer will be confirmed for finding a good condition and optimization within the crystal structure, the carrier transporting properties and the photovoltaic performance. The energy diagram and photovoltaic mechanisms will be discussed for optimization with improvement of the photovoltaic performance and optical absorption.
2. Results
The present photovoltaic cells of fluorine-doped tin-oxide (FTO) FTO/TiO
2/CH
3NH
3PbI
3−x−yBr
xCl
y/HTL/Au varied with molar ratio at
x,
y = 0, 1, 2, and 3 were shown in a schematic illustration in
Figure 1.
The perovskite solution varied with molar ratio of halogen doping was prepared as listed in
Table 1.
The
J-V characteristics of the perovskite solar cells varied with halogen doping material at certain percentages under illumination are shown in
Figure 2.
The photocurrent was observed under illumination, and the cell structure showed characteristic curves with regard to the short-circuit current density (
Jsc) and open-circuit voltage (
Voc).The detailed parameters of the best device are listed in
Table 2. The performance of
Voc was increased from 0.69 V to 0.74 V. The short-circuit current density,
Jsc, was increased from 14.3 mA·cm
−2 to 16.2 mA·cm
−2. Overall power conversion efficiency (
PCE) was improved from 2.7% to 7.0%. Incorporation of halogen doping of Cl and Br as dopant into the perovskite layer improved the photovoltaic performance of
Voc, Jsc, fill factor (
FF) and
PCE due to optimization of the band gap, extension of carrier diffusion, lifetime, promotion of carrier generation and charge separation with inhibiting carrier recombination in the microstructure. As a standard reference, fabrication and characterization of the reference for halogen doping I in the perovskite structure was repeatedly performed for confirmation of reproducibility. The low conversion seen in producing the sub-reaction of PbI
2 could be caused by degradation with hydrolysis reaction under humidity. Exposure of the perovskite crystal to light and humidity results in the decomposition to PbI
2. While the film preparation and measurements of the
J-V curve for halogen doping I, Cl and Br in the perovskite solar cells was performed under atmosphere conditions, the photovoltaic parameters of
Jsc, Voc, FF and
PCE were influenced by the film preparation conditions at normal humidity, such as 40%–60%. The effect of atmospheric humidity on the photovoltaic performance, carrier generation, charge separation and crystallization in the perovskite films has been investigated [
30]. The performance of
Voc and
FF with a low efficiency of conversion for halogen doping I in the perovskite solar cell derives from degradation of the crystal growth with carrier loss and leak current near the interface between the crystal domains in microstructure. Optimization of the perovskite crystal growth under the film preparation in a globe box filled with a high purity inert gas will improve the photovoltaic performance of
Jsc,
Voc,
FF,
PCE and incident photon to current conversion efficiency (IPCE).
Transmission spectra and Tauc-plot of the perovskite solar cells varied with halogen doping of I, Cl and Br as dopants are shown in
Figure 3. In the standard condition using halogen doping of I, the absorption of the solar cell was covered in the range of 300–830 nm. Incorporation of halogen doping using Cl and Br as dopants provided a slight drop of absorbance and blue-shift in a wide range of 300–800 nm. While a mixed halogen doping of I, Cl, and Br was added in the perovskite structure, the transmission absorbance decreased with decreasing concentration of I in the perovskite structure. Addition of Cl and Br in the perovskite structure improved the performance of the short-circuit current density,
Voc,
FF and
PCE for the devices. Introduction of halogen doping with Cl and Br in the perovskite structure optimized the carrier concentration in the perovskite structure, which improved the range of wavelength, the band gap, photo generation and carrier transporting properties and controlled the leakage current.
The optical energy gap as direct transition was estimated by Tauc-plot. As listed in
Table 3, the optical energy gap corresponding to wavelength was obtained to be 1.53 eV and 810 nm in the standard case using halogen doping of I. The optical energy gap was obtained to about 1.60 eV by addition of the halogen doping of I, Cl and Br. Incorporation with a small amount of halogen doping using I, Cl and Br increased the energy gap and carrier transporting behavior. The halogen doping of Cl and Br remarkably influenced the energy gap, wavelength and the photovoltaic performance of
Jsc,
Voc and
PCE. The role of chloride as dopant on the transporting behavior and the photovoltaic properties on perovskite hybrid solar cells has been discussed [
2]. Introducing halogen doping into the perovskite structure controlled the energy gap in the range of wavelengths, which provided the promotion of the photo generation and the carrier transporting properties in the active layer.
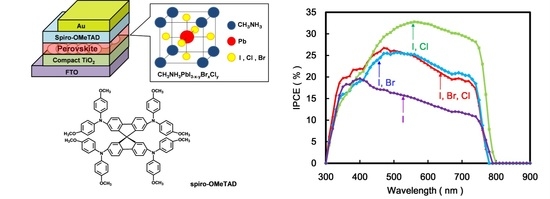
The IPCE of the perovskite solar cells with a small amount of halogen doping using I, Cl and Br compounds are shown in
Figure 4. In the standard case using halogen doping of I, the performance of IPCE in the solar cells was obtained to be 12%–18% in the range of 400–750 nm. When a mixture of two halogen doping of I and Br was used, IPCE slightly increased to be about 26% at 480 nm. In the case of two halogen doping with I and Cl, IPCE was increased to be 28%–32% in the range of 550–750 nm. When a mixture of I, Cl and Br was used as a three component system, IPCE slightly decreased to be about 25% at 480 nm. Introduction of halogen doping with I and Cl as dopants optimized the carrier concentration in the perovskite crystal structure for improving band gap, photo generation and carrier transporting properties and controlled the leakage current.
Figure 5 shows XRDs of the perovskite solar cells doped with I, Cl and Br. The diffraction patterns of CH
3NH
3PbI
3, PbI
2 and TiO
2 were identified as remarks. Under standard conditions of halogen doping of I, a strong diffraction peak in 2θ was obtained to be 14.07°, corresponding to be about 6.29 Å in
d-spacing. The spacing was assigned to the (100) surface of perovskite crystal structure.
Table 4 lists the diffraction parameters of 100 of the perovskite crystal structure doped by I, Cl and Br. The lattice constant was slightly decreased by a mixture of halogen doping of I, Cl and Br. The spacing was decreased by addition of Cl and Br compounds as compared with I. The crystallite size increased from 387 Å to 486 Å by addition of the halogen doping using I, Cl and Br. The crystal growth, size and crystal spacing in the perovskite structure was changed by addition of a small amount of halogen doping. Incorporation of I and Cl as dopants into the perovskite crystalline structure dramatically improved the photovoltaic performance, electronic structure, optical absorption, band gap and the transporting behavior within the perovskite layer. Control of chlorine and bromide as dopant is important to optimize the condition of the photo generation, carrier transporting behavior and the photovoltaic performance of
Jsc and
PCE.
The SEM images of surface morphologies of the perovskite solar cells doped with I, Cl and Br are shown in
Figure 6. In the standard case, as shown in
Figure 6a, the crystal nucleation in the perovskite layer is caused by the TiO
2 mesoporous surface layer. When a mixture of halogen doping using I, Cl and Br as dopants was added, the perovskite crystal growth gradually accelerated to about 10 μm in diameter on the mesoporous TiO
2 surface layer. From experimental results using XRD and SEM observation, the control of halogen doping condition in the perovskite structure is important to find the best condition for optimization of the crystal growth, the carrier transport properties and the photovoltaic performance.
Atomic composition on the perovskite layer in the solar cells was analyzed by energy dispersive EDX. The atomic compositions of Pb, I, Cl, and Br in the added case using I, Cl and Br are listed in
Table 5. In the standard case using I, the atomic composition of Pb and I was obtained to be 31.0% and 69.0%, yielding molar ratio of 1.0–2.2, which was slightly decreased as compared with halogen doping of Pb and I at molar ratio of 1–3. From the experimental results using the atomic composition of halogen doping using (I, Cl), (I, Br), (I, Cl, Br) as component systems, the molar ratio of Pb to I, Cl and Br was obtained to be 1.00:2.01:0.05:0, 1.00:2.06:0:0.06, and 1.00:1.82:0.05:0.06, respectively. When a mixture of halogen doping of I, Cl, and Br was added as two or three component systems, the halogen doping of Cl and Br diffused in the crystal structure and promoted the crystal growth. This result suggests improvement of the carrier transporting property and the photovoltaic performance of
Jsc and
PCE by the halogen doping into the perovskite crystal structure.
3. Discussion
The photovoltaic mechanism of the CH
3NH
3PbI
3−x−yBr
xCl
y perovskite solar cells is discussed on the basis of the energy diagram shown in
Figure 7. Light irradiation induced the generating and charge separation in CH
3NH
3PbI
3−x−yBr
xCl
y as the active layer. The excited electrons of CH
3NH
3PbI
3−x−yBr
xCl
y in the conduction band diffused and charges were transferred from CH
3NH
3PbI
3−x−yBr
xCl
y to TiO
2 onto the active layer at a FTO substrate. In addition, holes in valence band went through spiro-OMeTAD as HTL and arrived at the Au electrode as the cathode. An energy barrier would exist at the semiconductor–metal interface. The
Voc of the perovskite solar cells could be related to the energy gap between the valence band of CH
3NH
3PbI
3−x−yBr
xCl
y and conduction band of TiO
2. The energy levels of the HOMO, band gap and the carrier mobility of electron transporting layer could be controlled by combination of hydrogen doping of I, Cl and Br with molar ratio in the perovskite crystal structure [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. Incorporation of Cl and Br as dopants into the perovskite crystalline structure improved the crystal growth and size, the carrier transporting properties and the photovoltaic performance of
Voc, Jsc and
PCE. The electron mobility of the perovskite single crystals was 2.5 cm
2·V
−1·s
−1 [
11]. The carrier mobility of the perovskite layer depended on the variable amount of hydrogen doping of I, Cl and Br. It also varied with molar ratio. When the plural halogen doping using I, Cl and Br was used as a two or three component system, the photovoltaic performance, optical properties and band gap were improved as compared with single halogen doping of I.
The control of carrier diffusion, band gap between the energy levels at the conduction and valence bands in the CH3NH3PbI3−x−yBrxCly layer is an important factor to improve photovoltaic performance. From the energy diagram and photovoltaic mechanism of the perovskite solar cells, it can be seen that halogen doping of I, Cl and Br in the active layer caused carrier generation, carrier transfer properties and functions, partially because of the electron-transporting layer, improved current density, band gap and conversion efficiency. The photovoltaic performance depends on band gap, carrier mobility and carrier recombination around the interface between the perovskite layer and TiO2 mesoporous structure as the electron-transporting layer.
In fact, the doping conditions with a small amount of I, Cl and Br in the perovskite layer influenced the crystal spacing and growth of the perovskite structure for improving the carrier diffusion without recombination. The crystalline perovskite structure maintained the photovoltaic and optical properties by preventing the formation of a leakage current path around the interface. The halogen doping of I, Cl and Br into the perovskite layer have important influences on carrier transporting behavior and the perovskite crystal growth for improving the photovoltaic performance of Voc, Jsc, FF and PCE.
4. Materials and Methods
FTO-coated glass substrates were cleaned in an ultrasonic bath with acetone and methanol and dried under nitrogen gas. The 0.15 M TiO
2 precursor solution was prepared from titanium diisopropoxide bis(acetylacetonate) (55 μL, Sigma-Aldrich, Tokyo, Japan) in 1-butanol (1 mL), and the TiO
2 precursor solution was spin-coated on the FTO substrate at 3000 rpm for 30 s and annealed 125 °C for 5 min. Afterwards, the coated film was cooled to room temperature, the same process was repeated twice with 0.3 M titanium diisopropoxide bis(acetylacetonate) solution in 1-butanol at 3000 rpm. The coated FTO glasses with TiO
2 precursor solutions were heated at 500 °C for 30 min to form the compact TiO
2 layer. A mesoporous TiO
2 layer was deposited by spin-coating at 5000 rpm for 30 s. TiO
2 paste was prepared with TiO
2 powder (Aerosil P-25, Nippon Aerosil Co. Ltd., Tokyo, Japan) with poly(ethylene glycol) (PEG # 20000, Nacalai Tesque, Inc., Kyoto, Japan) in ultrapure water. The solution was mixed with acetyl acetone (10.0 μL, Wako Pure Chemical Industries, Osaka, Japan) and Triton X-100 (5 μL, Sigma-Aldrich, Tokyo, Japan) for 30 min. The layers were annealed at 500 °C for 30 min. For the preparation of pigment with a perovskite structure, a solution of methyl ammonium iodide (CH
3NH
3I) (Showa Chemical Co. Ltd., Tokyo, Japan), methyl ammonium chloride: (CH
3NH
3Cl) (Showa Chemical Co. Ltd., Tokyo, Japan), methyl ammonium bromide: (CH
3NH
3Br) (Showa Chemical Co. Ltd., Tokyo, Japan) and PbI
2 in γ-butyrolactone (Nacalai Tesque, Inc. Kyoto, Japan) was mixed at 70 °C as listed in
Table 1. The solution was then introduced into the TiO
2 mesoporous structure by spin-coating method and annealed at 100 °C for 15 min. As preparation of HTL, a solution of spiro-OMeTAD (36.1 mg, Wako Pure Chemical Industries, Osaka, Japan) in chlorobenzene (0.5 mL, Wako Pure Chemical Industries, Osaka, Japan) was mixed with a solution of lithium bis(trifluoromethylsulfonyl) imide (Li-TFSI, 260 mg, Tokyo Chemical Industry, Tokyo, Japan) in acetonitrile (0.5 mL, Nacalai Tesque, Inc., Kyoto, Japan) for 12 h. The former solution with 4-tert-butylpyridine (14.4 μL Sigma-Aldrich, Tokyo, Japan) was mixed with the Li-TFSI solution (8.8 μL) for 30 min at 70 °C. The HTL solution was prepared on the perovskite structure layer deposed on TiO
2/FTO layer by spin coating. The gold (Au) top electrode was thermally evaporated through a stainless steel mask with an area of 0.090 cm
2 (0.3 cm × 0.3 cm). Layered structures of the present photovoltaic cells were denoted as FTO/TiO
2/CH
3NH
3PbI
3−x−yBr
xCl
y/HTL/Au, as shown in a schematic illustration in
Figure 1.
The
J-V characteristics of the photovoltaic solar cells were measured both in the dark and under the illumination at 100 mW·cm
−2 (AM 1.5 air mass) using a solar simulator (XES-301S, San-ei Electric Co. Ltd., Osaka, Japan) and potentiostat (HSV-110, Hokuto Denko Corporation, Tokyo, Japan). The masked solar cell was 0.090 cm
2 (0.3 cm × 0.3 cm). The photocurrent was observed under illumination, and the cell structure showed characteristic curves with regard to the short-circuit current density (
Jsc) and open-circuit voltage (
Voc). The experimental parameters of forward scan rate, starting and last voltage were fixed to be 100 mV·s
−1, −0.2 V and 1.0 V, respectively. Without holding at constant time of the device under illumination in ambient condition, the once scanning of
J-V curve measurement was performed in the range of forward voltage from −0.2 V to 1.0 V. The hysteresis measurement of photocurrent density with forward and backward voltage was not performed for investigating the transient phenomena. The photovoltaic characterization depended on the film preparation and measurement conditions [
31]. Repeated measurements of
J-V curve were performed for obtaining an average value. The detailed parameters of the best device are listed in
Table 2.
Optical absorption properties were investigated by means of ultraviolet-visible-near-infrared spectroscopy (Jasco V-670, Jasco Corporation, Tokyo, Japan). Optical band gap as direct transition of the perovskite solar cells doped with I, Cl and Br was estimated by Tauc-plot. The X-ray diffraction measurements were performed and equipped with a monochromatic CuKα X-ray source (λ = 0.15418 nm) (Bruker D2 PHASER, Bruker Corporation, Billerica, MA, USA). Surface morphology was observed by optical microscopy (Nikon eclipse E600, Nikon Corporation, Tokyo, Japan) and analytical SEM (JEOL JSM-6010 PLUS/LA, JEOL Ltd., Tokyo, Japan). Atomic composition on the perovskite layer in the solar cells was analyzed by energy dispersive EDX. The external quantum efficiency (EQE) values of the solar cells (Enlitech QE-R3011, Enlitech Technology Co. Ltd., Kaohsiung, Taiwan) were measured. The device active area for all the solar cells was 0.090 cm2.
5. Conclusions
Fabrication and characterization of CH3NH3PbI3−x−yBrxCly perovskite solar cells using mesoporous TiO2 as the electronic transporting layer and spiro-OMeTAD as the HTL were carried out. The effect of halogen doping using I, Br and Cl compounds as dopant on the photovoltaic, optical properties and crystal structures of the perovskite-based solar cells was investigated. When a mixture of halogen dopants using I, Cl and Br was added into the perovskite structure, the photovoltaic performance of Jsc, Voc, FF, PCE, optical wavelength and crystalline growth behavior were improved in comparison with other cases using single halogen doping with I, Cl and Br. The XRD confirmed a slight decrease of lattice constant of the perovskite structure doped with I, Br, and Cl in the solar cell. SEM and energy dispersive EDX identified the crystalline growth behavior by enhancing the crystal size, and determined molar ratio of Pb, I, Br and Cl in the CH3NH3PbI3−x−yBrxCly perovskite structure. Incorporation of the halogen doping using Pb, I, Br and Cl in the crystal structure promoted the photo generation and carrier diffusion with suppressing carrier recombination in the crystal structure, and optimized the electron structure with a slight change of band gap, improving the photovoltaic performance of Voc, Jsc and IPCE. The photovoltaic mechanism of the perovskite solar cells was discussed in the context of the experimental results.