Zinc Porphyrins Possessing Three p-Carboxyphenyl Groups: Effect of the Donor Strength of Push-Groups on the Efficiency of Dye Sensitized Solar Cells
Abstract
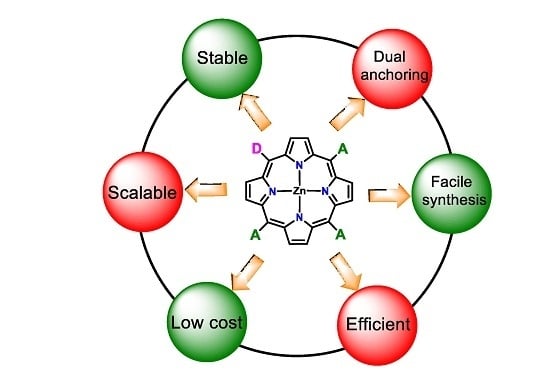
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Photophysical Properties
2.3. Electrochemical Properties
2.4. Quantum Chemical Results
2.5. Photovoltaic Properties
2.6. Stability Test of Porphyrin Sensitizers
3. Materials and Methods
3.1. Synthesis
3.2. Optical Spectroscopy
3.3. Cyclic Voltammetry
3.4. Density Functional Theory Calculations
3.5. Photovoltaic Measurements
3.6. Stability Test of Porphyrin Sensitizers
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.M.; Burrell, A.K.; Officer, D.L.; Jolley, K.W. Porphyrins as light harvesters in the dye-sensitised TiO2 solar cell. Coord. Chem. Rev. 2004, 248, 1363–1379. [Google Scholar] [CrossRef]
- Imahori, H.; Umeyama, T.; Ito, S. Large π-aromatic molecules as potential sensitizers for highly efficient dye-sensitized solar cells. Acc. Chem. Res. 2009, 42, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Diaz, M.V.; de la Torre, G.; Torres, T. Lighting porphyrins and phthalocyanines for molecular photovoltaics. Chem. Commun. 2010, 46, 7090–7108. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Rudine, A.B.; Wamser, C.C. Porphyrins and phthalocyanines in solar photovoltaic cells. J. Porphyr. Phthalocyanines 2010, 14, 759–792. [Google Scholar] [CrossRef]
- Li, L.L.; Diau, E.W.G. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Bessho, T.; Zakeeruddin, S.M.; Yeh, C.Y.; Diau, E.W.G.; Grätzel, M. Highly efficient mesoscopic dye-sensitized solar cells based on donor–acceptor-substituted porphyrins. Angew. Chem. Int. Ed. 2010, 49, 6646–6649. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; CurchodBasile, F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Yella, A.; Lee, H.W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.G.; Yeh, C.Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Yella, A.; Mai, C.L.; Zakeeruddin, S.M.; Chang, S.N.; Hsieh, C.H.; Yeh, C.Y.; Grätzel, M. Molecular engineering of push-pull porphyrin dyes for highly efficient dye-sensitized solar cells: The role of benzene spacers. Angew. Chem. Int. Ed. 2014, 53, 2973–2977. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Islam, A.; Chen, H.; Malapaka, C.; Chiranjeevi, B.; Zhang, S.; Yang, X.; Yanagida, M. High-efficiency dye-sensitized solar cell with a novel co-adsorbent. Energy Environ. Sci. 2012, 5, 6057–6060. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Otsuka, T.; Kyomen, T.; Unno, M.; Hanaya, M. An achievement of over 12 percent efficiency in an organic dye-sensitized solar cell. Chem. Commun. 2014, 50, 6379–6381. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.B.; Cheng, C.F.; Sutanto, A.A.; Datta, A.; Dutta, A.; Hung, C.H. DA-π-A organic dyes for dye-sensitized solar cells: Effect of π-bridge length between two acceptors on photovoltaic properties. Tetrahedron 2015, 71, 7977–7984. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, Y.; Wu, W.; Wang, Y.; Liu, J.; Li, X.; Tian, H.; Zhu, W.H. Porphyrin cosensitization for a photovoltaic efficiency of 11.5%: A record for non-ruthenium solar cells based on iodine electrolyte. J. Am. Chem. Soc. 2015, 137, 14055–14058. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Murakoshi, K.; Kano, G.; Wada, Y.; Yanagida, S.; Miyazaki, H.; Matsumoto, M.; Murasawa, S. Importance of binding states between photosensitizing molecules and the TiO2 surface for efficiency in a dye-sensitized solar cell. J. Electroanal. Chem. 1995, 396, 27–34. [Google Scholar] [CrossRef]
- Clifford, J.N.; Palomares, E.; Nazeeruddin, M.K.; Grätzel, M.; Nelson, J.; Li, X.; Long, N.J.; Durrant, J.R. Molecular control of recombination dynamics in dye-sensitized nanocrystalline TiO2 films: Free energy vs. distance dependence. J. Am. Chem. Soc. 2004, 126, 5225–5233. [Google Scholar] [CrossRef] [PubMed]
- Durrant, J.R.; Haque, S.A.; Palomares, E. Towards optimisation of electron transfer processes in dye sensitised solar cells. Coord. Chem. Rev. 2004, 248, 1247–1257. [Google Scholar] [CrossRef]
- Palomares, E.; Martinez-Diaz, M.V.; Haque, S.A.; Torres, T.; Durrant, J.R. State selective electron injection in non-aggregated titanium phthalocyanine sensitised nanocrystalline TiO2 films. Chem. Commun. 2004, 18, 2112–2113. [Google Scholar] [CrossRef] [PubMed]
- Ladomenou, K.; Kitsopoulos, T.N.; Sharma, G.D.; Coutsolelos, A.G. The importance of various anchoring groups attached on porphyrins as potential dyes for DSSC applications. RSC Adv. 2014, 4, 21379–21404. [Google Scholar] [CrossRef]
- Abbotto, A.; Manfredi, N.; Marinzi, C.; de Angelis, F.; Mosconi, E.; Yum, J.-H.; Xianxi, Z.; Nazeeruddin, M.K.; Grätzel, M. Di-branched di-anchoring organic dyes for dye-sensitized solar cells. Energy Environ. Sci. 2009, 2, 1094–1101. [Google Scholar] [CrossRef]
- Garcia-Iglesias, M.; Yum, J.-H.; Humphry-Baker, R.; Zakeeruddin, S.M.; Pechy, P.; Vazquez, P.; Palomares, E.; Gratzel, M.; Nazeeruddin, M.K.; Torres, T. Effect of anchoring groups in zinc phthalocyanine on the dye-sensitized solar cell performance and stability. Chem. Sci. 2011, 2, 1145–1150. [Google Scholar] [CrossRef]
- Ma, T.; Inoue, K.; Yao, K.; Noma, H.; Shuji, T.; Abe, E.; Yu, J.; Wang, X.; Zhang, B. Photoelectrochemical properties of TiO2 electrodes sensitized by porphyrin derivatives with different numbers of carboxyl groups. J. Electroanal. Chem. 2002, 537, 31–38. [Google Scholar] [CrossRef]
- Odobel, F.; Blart, E.; Lagree, M.; Villieras, M.; Boujtita, H.; el Murr, N.; Caramori, S.; Alberto Bignozzi, C. Porphyrin dyes for TiO2 sensitization. J. Mater. Chem. 2003, 13, 502–510. [Google Scholar] [CrossRef]
- Gou, F.; Jiang, X.; Fang, R.; Jing, H.; Zhu, Z. Strategy to improve photovoltaic performance of dssc sensitized by zinc prophyrin using salicylic acid as a tridentate anchoring group. ACS Appl. Mater. Interfaces 2014, 6, 6697–6703. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, T.; Arai, Y.; Segawa, H. Porphyrins with β-acetylene-bridged functional groups for efficient dye-sensitized solar cells. RSC Adv. 2014, 4, 13201–13204. [Google Scholar] [CrossRef]
- Ishida, M.; Hwang, D.; Zhang, Z.; Choi, Y.J.; Oh, J.; Lynch, V.M.; Kim, D.Y.; Sessler, J.L.; Kim, D. β-functionalized push-pull porphyrin sensitizers in dye-sensitized solar cells: Effect of π-conjugated spacers. ChemSusChem 2015, 8, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Qian, X.; Zhang, P.F.; Zhu, Y.Z.; Zheng, J.Y. A meso-meso directly linked porphyrin dimer-based double D-π-A sensitizer for efficient dye-sensitized solar cells. Chem. Commun. 2015, 51, 3782–3785. [Google Scholar] [CrossRef] [PubMed]
- Higashino, T.; Fujimori, Y.; Sugiura, K.; Tsuji, Y.; Ito, S.; Imahori, H. Synthesis of push-pull porphyrin with two electron-donating and two electron-withdrawing groups and its application to dye-sensitized solar cell. J. Porphyr. Phthalocyanines 2015, 19, 140–149. [Google Scholar] [CrossRef]
- Ambre, R.; Yu, C.Y.; Mane, S.B.; Yao, C.F.; Hung, C.H. Toward carboxylate group functionalized A4, A2B2, A3B oxaporphyrins and zinc complex of oxaporphyrins. Tetrahedron 2011, 67, 4680–4688. [Google Scholar] [CrossRef]
- Ambre, R.; Chen, K.B.; Yao, C.F.; Luo, L.; Diau, E.W.G.; Hung, C.H. Effects of porphyrinic meso-substituents on the photovoltaic performance of dye-sensitized solar cells: Number and position of p-carboxyphenyl and thienyl groups on zinc porphyrins. J. Phys. Chem. C 2012, 116, 11907–11916. [Google Scholar] [CrossRef]
- Mane, S.B.; Hu, J.Y.; Chang, Y.C.; Luo, L.; Diau, E.W.G.; Hung, C.H. Novel expanded porphyrin sensitized solar cells using boryl oxasmaragdyrin as the sensitizer. Chem. Commun. 2013, 49, 6882–6884. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.B.; Hung, C.H. Synthesis of carboxylate functionalized A3B and A2B2 thiaporphyrins and their application in dye-sensitized solar cells. New J. Chem. 2014, 38, 3960–3972. [Google Scholar] [CrossRef]
- Mane, S.B.; Luo, L.; Chang, G.F.; Diau, E.W.G.; Hung, C.H. Effects of core-modification on porphyrin sensitizers to the efficiencies of dye-sensitized solar cells. J. Chin. Chem. Soc. 2014, 61, 545–555. [Google Scholar] [CrossRef]
- Luo, L.; Ambre, R.B.; Mane, S.B.; Diau, E.W.G.; Hung, C.H. The cis-isomer performs better than the trans-isomer in porphyrin-sensitized solar cells: Interfacial electron transport and charge recombination investigations. Phys. Chem. Chem. Phys. 2015, 17, 20134–20143. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.B.; Hung, C.H. Molecular engineering of boryl oxasmaragdyrins through peripheral modification: Structure–efficiency relationship. Chem. A Eur. J. 2015, 21, 4825–4841. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.B.; Luo, L.; Tsai, H.H.; Hung, C.H. Co-sensitization of free-base and zinc porphyrins: An effective approach to improve the photon-to-current conversion efficiency of dye-sensitized solar cells. J. Porphyr. Phthalocyanines 2015, 19, 695–707. [Google Scholar] [CrossRef]
- Ambre, R.B.; Chang, G.F.; Zanwar, M.R.; Yao, C.F.; Diau, E.W.G.; Hung, C.H. New dual donor-acceptor (2d-π-2a) porphyrin sensitizers for stable and cost-effective dye-sensitized solar cells. Chem. Asian J. 2013, 8, 2144–2153. [Google Scholar] [CrossRef] [PubMed]
- Ambre, R.B.; Chang, G.F.; Hung, C.H. Three p-carboxyphenyl groups possessing zinc porphyrins: Efficient, stable, and cost-effective sensitizers for dye-sensitized solar cells. Chem. Commun. 2014, 50, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Ambre, R.B.; Mane, S.B.; Chang, G.F.; Hung, C.H. Effects of number and position of meta and para carboxyphenyl groups of zinc porphyrins in dye-sensitized solar cells: Structure–performance relationship. ACS Appl. Mater. Interfaces 2015, 7, 1879–1891. [Google Scholar] [CrossRef] [PubMed]
- Zervaki, G.E.; Roy, M.S.; Panda, M.K.; Angaridis, P.A.; Chrissos, E.; Sharma, G.D.; Coutsolelos, A.G. Efficient sensitization of dye-sensitized solar cells by novel triazine-bridged porphyrin-porphyrin dyads. Inorg. Chem. 2013, 52, 9813–9825. [Google Scholar] [CrossRef] [PubMed]
- Zervaki, G.E.; Papastamatakis, E.; Angaridis, P.A.; Nikolaou, V.; Singh, M.; Kurchania, R.; Kitsopoulos, T.N.; Sharma, G.D.; Coutsolelos, A.G. A propeller-shaped, triazine-linked porphyrin triad as efficient sensitizer for dye-sensitized solar cells. Eur. J. Inorg. Chem. 2014, 2014, 1020–1033. [Google Scholar] [CrossRef]
- Narra, V.K.; Ullah, H.; Singh, V.K.; Giribabu, L.; Senthilarasu, S.; Karazhanov, S.Z.; Tahir, A.A.; Mallick, T.K.; Upadhyaya, H.M. D-π-a system based on zinc porphyrin dyes for dye-sensitized solar cells: Combined experimental and DFT-TDDFT study. Polyhedron 2015, 100, 313–320. [Google Scholar] [CrossRef]









| Dye | λabs (nm) 1 | λem (nm) 2 | Eox (V) 3 | E0-0 (eV) 4 | Eox* (V) 5 |
|---|---|---|---|---|---|
| Zn1TH3A | 427 (350) | 612 | 0.93 | 2.04 | −1.11 |
| 558 (18) | 656 | - | - | - | |
| 600 (9) | - | - | - | - | |
| Zn1TC3A | 426 (409) | 605 | 0.94 | 2.06 | −1.12 |
| 558 (17) | 654 | - | - | - | |
| 599 (6) | - | - | - | - | |
| Zn1BC3A | 426 (452) | 606 | 0.91 | 2.06 | −1.15 |
| 557 (19) | 658 | - | - | - | |
| 598 (7) | - | - | - | - | |
| Zn1OD3A | 426 (391) | 605 | 1.06 | 2.06 | −1.00 |
| 557 (18) | 657 | - | - | - | |
| 597 (7) | - | - | - | - | |
| Zn1OH3A | 426 (471) | 607 | 0.97 | 2.06 | −1.09 |
| 557 (18) | 656 | 1.33 | - | - | |
| 597 (7) | - | - | - | - | |
| Zn1NP3A | 426 (248) | 621 | 0.94 | 2.03 | −1.09 |
| 560 (18) | - | 1.06 | - | - | |
| 604 (11) | - | - | - | - | |
| Zn1NH3A 6 | 425 (178) | 622 | 0.96 | 2.03 | −1.07 |
| 559 (13) | - | 1.08 | - | - | |
| 603 (8) | - | - | - | - | |
| Zn1NN3A | 425 (218) | 624 | 1.01 | 2.03 | −1.02 |
| 559 (17) | - | 1.10 | - | - | |
| 603 (11) | - | - | - | - | |
| Zn1ND3A 6 | 425(143) | 624 | 0.92 | 2.03 | −1.11 |
| 560 (12) | - | 1.05 | - | - | |
| 603 (8) | - | - | - |
| Dye | Jsc (mA·cm−2) | Voc (V) | FF (%) | η (%) | Г (nmol·cm−2) |
|---|---|---|---|---|---|
| Zn1TH3A | 12.41 | 0.65 | 67 | 5.36 | 102 |
| Zn1TC3A | 9.95 | 0.62 | 67 | 4.49 | 135 |
| Zn1BC3A | 10.45 | 0.62 | 68 | 4.40 | 132 |
| Zn1OD3A | 10.80 | 0.66 | 66 | 4.78 | 90 |
| Zn1OH3A | 11.98 | 0.63 | 64 | 4.81 | 125 |
| Zn1NP3A | 13.86 | 0.64 | 66 | 5.86 | 140 |
| Zn1NH3A | 14.55 | 0.68 | 65 | 6.50 | 138 |
| Zn1NN3A | 12.87 | 0.67 | 65 | 5.65 | 126 |
| Zn1ND3A | 13.50 | 0.67 | 66 | 6.00 | 137 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambre, R.B.; Mane, S.B.; Hung, C.-H. Zinc Porphyrins Possessing Three p-Carboxyphenyl Groups: Effect of the Donor Strength of Push-Groups on the Efficiency of Dye Sensitized Solar Cells. Energies 2016, 9, 513. https://doi.org/10.3390/en9070513
Ambre RB, Mane SB, Hung C-H. Zinc Porphyrins Possessing Three p-Carboxyphenyl Groups: Effect of the Donor Strength of Push-Groups on the Efficiency of Dye Sensitized Solar Cells. Energies. 2016; 9(7):513. https://doi.org/10.3390/en9070513
Chicago/Turabian StyleAmbre, Ram B., Sandeep B. Mane, and Chen-Hsiung Hung. 2016. "Zinc Porphyrins Possessing Three p-Carboxyphenyl Groups: Effect of the Donor Strength of Push-Groups on the Efficiency of Dye Sensitized Solar Cells" Energies 9, no. 7: 513. https://doi.org/10.3390/en9070513
APA StyleAmbre, R. B., Mane, S. B., & Hung, C. -H. (2016). Zinc Porphyrins Possessing Three p-Carboxyphenyl Groups: Effect of the Donor Strength of Push-Groups on the Efficiency of Dye Sensitized Solar Cells. Energies, 9(7), 513. https://doi.org/10.3390/en9070513