Impact of Drought and Salinity on Sweetgum Tree (Liquidambar styraciflua L.): Understanding Tree Ecophysiological Responses in the Urban Context
Abstract
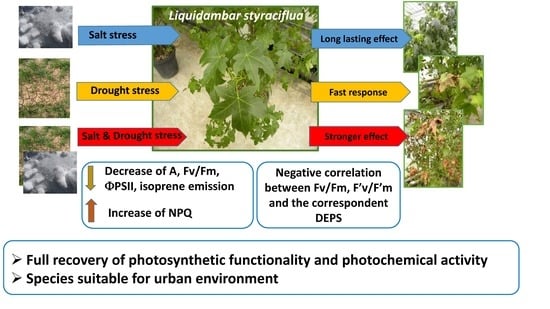
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Set-Up
2.2. Meteorological Measurements
2.3. Physiological Measurements
2.3.1. Leaf Water Potential Analysis
2.3.2. Gas Exchanges and Chlorophyll Fluorescence Measurements
2.3.3. Xanthophyll Analysis
2.3.4. Isoprene Analysis
2.4. Statistical Analyses
3. Results
3.1. Meteorological Data
3.2. Physiological Measurements
3.2.1. Plant Water Status
3.2.2. Photosynthesis and Chlorophyll Fluorescence Parameters
3.2.3. Xanthophyll Cycle
3.2.4. Isoprene Emission
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nowak, D.J.; Crane, D.E. Carbon storage and sequestration by urban trees in the USA. Environ. Pollut. 2002, 116, 381–389. [Google Scholar] [CrossRef]
- Gómez-Baggethun, E.; Barton, D.N. Classifying and valuing ecosystem services for urban planning. Ecol. Econ. 2013, 86, 235–245. [Google Scholar] [CrossRef]
- Nowak, D.J.; Greenfield, E.J.; Hoehn, R.; Lapoint, E. Carbon storage and sequestration by trees in urban and community areas of the United States. Environ. Pollut. 2013, 178, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, F.; Ferrini, F.; Pollastrini, M.; Fini, A. The challenge of Mediterranean schlerophyllous vegetation under climate change: From acclimation to adaptation. Environ. Exp. Bot. 2014, 103, 80–98. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Equiza, M.A.; Calvo-Polanco, M.; Cirelli, D.; Señorans, J.; Wartenbe, M.; Saunders, C.; Zwiazek, J.J. Long-term impact of road salt (NaCl) on soil and urban trees in Edmont, Canada. Urban For. Urban Green. 2017, 21, 16–28. [Google Scholar] [CrossRef]
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc. Natl. Acad. Sci. USA 2009, 106, 7063–7066. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Račková, L.; Paganova, V.; Swoczyna, T.; Rusinowski, S.; Sitko, K. Can chlorophyll-a fluorescence parameters be used as bio-indicators to distinguish drought and salinity stress in Tilia cordata Mill? Environ. Exp. Bot. 2018, 152, 149–157. [Google Scholar] [CrossRef]
- Čekstere, G.; Osvalde, A. A study of chemical characteristics of soil in relation to street trees status in Riga (Latvia). Urban For. Urban Green. 2013, 12, 69–78. [Google Scholar] [CrossRef]
- Godwin, K.S.; Hafner, S.D.; Buff, M.F. Long-trend trends in sodium and chloride in the Mohawk River. New York: The effect of fifty years of road-salt application. Environ. Pollut. 2003, 124, 273–281. [Google Scholar] [CrossRef]
- Ramakrishna, D.M.; Viraraghavan, T. Environmental impact of chemical deicers—A review. Water Air Soil Pollut. 2005, 166, 49–63. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, S.; Zhao, H.; Korpelainen, H.; Li, C. Sex-related adaptive responses to interaction of drought and salinity in Populus yunnanensis. Plant Cell Environ. 2010, 33, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stress on plant growth and yield: A review. Int. Agrophys. 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Lui, J.; Shi, D.C. Photosynthesis, chlorophyll fluorescence, inorganic ion and organic acid accumulations of sunflower in responses to salt and salt-alkaline mixed stress. Photosynthetica 2010, 48, 127–134. [Google Scholar]
- Mehta, P.; Allakhverdiev, S.I.; Jajoo, A. Characterization of photosystem II heterogeneity in response to high salt stress in wheat leaves (Triticum aestivum). Photosynth. Res. 2010, 105, 249–255. [Google Scholar] [CrossRef]
- Tsonev, T.; Wahbi, S.; Sun, P.; Sorreutiuo, G.; Centritto, M. Gas exchange, water relations photochemical reflectance index in stress and recovery and their relationships with Quercus ilex plants during water stress and recovery. Int. J. Agric. Biol. 2014, 16, 335–341. [Google Scholar]
- Szabo, I.; Bergantino, E.; Giacometti, G.M. Light and oxygenic photosynthesis: Energy dissipation as a protection mechanism against photo-oxidation. EMBO Rep. 2005, 6, 629–634. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig-Adams, B. Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 17–47. [Google Scholar]
- Müller, P.; Li, X.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Jahns, P.; Holzwarth, A.R. The role of xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Munnè-Bosch, S.; Alegre, L. The xanthophyll cycle is induced by light irrespective of water status in field-grown lavender (Lavandula stoechas) plants. Physiol. Plant 2000, 108, 147–151. [Google Scholar] [CrossRef]
- Baraldi, R.; Canaccini, F.; Cortes, S.; Magnani, F.; Rapparini, F.; Zamboni, A.; Raddi, S. Role of xanthophyll cycle-mediated photoprotection in Arbutus unedo plants exposed to water stress during the Mediterranean summer. Photosynthetica 2008, 46, 378–386. [Google Scholar] [CrossRef]
- Latowski, D.; Kuczynska, P.; Strzalka, K. Xanthophyll cycle—A mechanism protecting plants against oxidative stress. Redox Rep. 2011, 16, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Munnè-Bosch, S.; Peñuelas, J. Photo-and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 2013, 217, 758–766. [Google Scholar] [CrossRef]
- Rapparini, F.; Neri, L.; Mihailova, G.; Petkova, S.; Georgieva, K. Growth irradiance affects the photoprotective mechanisms of the resurrection angiosperm Haberlea rhodopensis Friv. in response to desiccation and rehydration at morphological, physiological and biochemical levels. Environ. Exp. Bot. 2015, 113, 67–79. [Google Scholar] [CrossRef]
- Velikova, V.; Loreto, F. On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ. 2005, 28, 318–327. [Google Scholar] [CrossRef]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Lahr, E.C.; Schade, G.W.; Crossett, C.C.; Watson, M.R. Photosynthesis and isoprene emission from trees along an urban-rural gradient in Texas. Glob. Chang. Biol 2015, 21, 4221–4236. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Sun, Z. How light, temperature, and measurement and growth [CO2] interactively control isoprene emission in hybrid aspen. J. Exp. Bot. 2014, 66, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Fineschi, S. Reconciling functions and evolution of isoprene emission in higher plants. New Phytol. 2015, 206, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Arneth, A.; Kuhn, U.; Monson, R.K.; Peñuelas, J.; Staudt, M. The emission factor of volatile isoprenoids: Stress, acclimation, and developmental responses. Biogeosciences 2010, 7, 2203–2223. [Google Scholar] [CrossRef] [Green Version]
- Funk, J.L.; Jones, C.G.; Gray, D.W.; Throop, H.L.; Hyatt, L.A.; Lerdau, M.T. Variation in isoprene emission from Quercus rubra: Sources, causes, and consequences for estimating fluxes. J. Geophys. Res. 2005, 110, D04301. [Google Scholar] [CrossRef] [Green Version]
- Brilli, F.; Barta, C.; Fortunati, A.; Lerdau, M.; Loreto, F.; Centritto, M. Response of isoprene and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol. 2007, 175, 244–254. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Clemitshaw, K.C. Ozone and other secondary photochemical pollutants: Chemical processes governing their formation in the planetary boundary layer. Atmos. Environ. 2000, 34, 2499–2527. [Google Scholar] [CrossRef]
- Tsui, J.K.; Guenther, A.; Yip, W.K.; Chen, F. A biogenic volatile organic compound emission inventory for Hong Kong. Atmos. Environ. 2009, 43, 6442–6448. [Google Scholar] [CrossRef] [Green Version]
- Slama, I.; Ghnaya, T.; Savouré, A.; Abdelly, C. Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation of Sesuvium portulacastrum. C. R. Biol. 2008, 331, 442–451. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combination. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Brown, C.E.; Pezeshki, S.R.; DeLaune, R.D. The effects of salinity and soil drying on nutrient uptake and growth of Spartina alterniflora in a simulated tidal system. Environ. Exp. Bot. 2006, 58, 140–148. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Guo, X.; Harivandi, A. Salt tolerance and salt accumulation of landscape plants irrigated by sprinkler and drip irrigation systems. J. Plant Nutr. 2001, 24, 1473–1490. [Google Scholar] [CrossRef]
- Esperón-Rodríguez, M.; Barradas, V.L. Ecophysiological vulnerability to climate change: Water stress responses in four tree species from the central mountain region of Veracruz, Mexico. Reg. Environ. Chang. 2015, 15, 93–108. [Google Scholar] [CrossRef]
- Guenther, A.P.; Zimmerman, P.; Harley, R.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Model evaluation and sensitivity analysis. J. Geophys. Res. 1993, 98, 12609–12617. [Google Scholar] [CrossRef] [Green Version]
- Ripullone, F.; Rivelli, A.R.; Baraldi, R.; Guarini, R.; Guerrieri, R.; Magnani, F.; Peñuelas, J.; Raddi, S.; Borghetti, M. Effectiveness of the photochemical reflectance index to track photosynthetic activity over a range of forest tree species and plant water statuses. Funct. Plant Biol. 2011, 38, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Hernandez, I.; Alegre, L.; Munnè-Bosch, S. Enhanced α-tocopherol quinone levels and xanthophyll cycle de-epoxidation in rosemary plants exposed to water deficit during a Mediterranean winter. J. Plant Physiol. 2006, 163, 601–606. [Google Scholar] [CrossRef]
- Baraldi, R.; Neri, L.; Costa, F.; Facini, O.; Rapparini, F.; Carriero, G. Ecophysiological and micromorphological characterization of green roof vegetation for urban mitigation. Urban For. Urban Green. 2019, 37, 24–32. [Google Scholar] [CrossRef]
- Čekstere, G.; Nikodemus, O.; Osvalde, A. Toxic impact of the de-icing material to street greenery in Riga, Latvia. Urban For. Urban Green. 2008, 7, 207–217. [Google Scholar] [CrossRef]
- Savi, T.; Bertuzzi, S.; Branca, S.; Tretiach, M.; Nardini, A. Drought-induced xylem cavitation and hydraulic deterioration: Risk factors for urban trees under climate change? New Phytol. 2015, 205, 1106–1116. [Google Scholar] [CrossRef] [Green Version]
- Leibar, U.; Aizpurua, A.; Unamunzaga, O.; Pascual, I.; Morales, F. How will climate change influence grapevine cv. Tempranillo photosynthesis under different soil textures? Photosynth. Res. 2015, 124, 199–215. [Google Scholar] [CrossRef]
- Zhang, C.; Preece, C.; Filella, I.; Farrè-Armengol, G.; Peñuelas, J. Assessment of the response of photosynthetic activity of Mediterranean evergreen oaks to enhanced drought stress and recovery by using PRI and R690/R630. Forests 2017, 8, 386. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Zheng, S.; Zhong, Y.; Shangguan, Z. Contrasting dynamics of leaf potential and gas exchange during progressive drought cycles and recovery in Amorpha fruticosa and Robinia pseudoacacia. Sci. Rep. 2017, 7, 4470. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Levizou, E.; Petropoulou, E.; Manetas, Y. Carotenoid composition of peridermal twigs does not fully conform to a shade acclimation hypotesis. Photosynthetica 2004, 42, 591–596. [Google Scholar] [CrossRef]
- Benjamin, M.T.; Winer, A.M. Estimating the ozone-forming potential of urban trees and shrubs. Atmos. Environ. 1998, 32, 53–68. [Google Scholar] [CrossRef]
- Fang, C.; Monson, R.K.; Cowling, E.B. Isoprene emission, photosynthesis, and growth in sweetgum (Liquidambar styraciflua) seedlings exposed to short-and long-term drying cycles. Tree Physiol. 1996, 16, 441–446. [Google Scholar] [CrossRef]
- Harley, P.; Guenther, A.; Zimmerman, P. Effects of light, temperature and canopy position on net photosynthesis and isoprene emission from sweetgum (Liquidambar styraciflua) leaves. Tree Physiol. 1996, 16, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, Ü.; Tenhunen, J.D.; Harley, P.C.; Steinbrecher, R. A model of isoprene emission based on energetic requirements for isoprene synthesis and leaf photosynthetic properties for Liquidambar and Quercus. Plant Cell Environ. 1999, 22, 1319–1335. [Google Scholar] [CrossRef]
- Fortunati, A.; Barta, C.; Brilli, F.; Centritto, M.; Zimmer, I.; Schnitzler, J.P.; Loreto, F. Isoprene emission is not temperature dependent during and after severe drought stress: A physiological and biochemical analysis. Plant J. 2008, 55, 687–697. [Google Scholar] [CrossRef]
- Centritto, M.; Brilli, F.; Fodale, R.; Loreto, F. Different sensitivity of isoprene emission, respiration and photosynthesis to high growth temperature coupled with drought stress in black poplar (Populus nigra) saplings. Tree Physiol. 2011, 31, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Lavoir, A.V.; Staudt, M.; Schnitzler, J.P.; Landais, D.; Massol, F.; Rocheteau, A.; Rodriguez, R.; Zimmer, I.; Rambal, S. Drought reduced monoterpene emissions from the evergreen Mediterranean oaks Quercus ilex: Results from a throughfall displacement experiment. Biogeoscienses 2009, 6, 1167–1180. [Google Scholar] [CrossRef] [Green Version]
- Ghirardo, A.; Gutknecht, J.; Zimmer, I.; Brüggemann, N.; Schnitzler, J.P. Biogenic volatile organic compound and respiratory CO2 emission after 13C-labelling: Online tracing of C translocation dynamics in poplar plants. PLoS ONE 2011, 6, e17393. [Google Scholar] [CrossRef] [Green Version]
- Fall, R.; Monson, R.K. Isoprene emission rate and intercellular isoprene concentration as influenced by stomatal distribution. Plant Physiol. 1992, 100, 987–992. [Google Scholar] [CrossRef] [Green Version]
- Morfopoulos, C.; Sperlich, D.; Peñuelas, J.; Filella, I.; Llusià, J.; Medlyn, B.E.; Niinemets, U.; Possell, M.; Sun, Z.; Prentice, I.C. A model of plant isoprene emission based on available reducing power captures responses to atmospheric CO2. New Phytol. 2014, 203, 125–139. [Google Scholar] [CrossRef]
- Loreto, F.; Pinelli, P.; Brancaleoni, E.; Ciccioli, P. 13C labeling reveals chloroplastic and extrachloroplastic pools of dimethylallyl pyrophosphate and their contribution to isoprene formation. Plant Physiol. 2004, 135, 1903–1907. [Google Scholar] [CrossRef] [Green Version]
- Beckett, M.; Loreto, F.; Velikova, V.; Brunetti, C.; di Ferdinando, M.; Tattini, M.; Calfapietra, C.; Farrant, J.M. Photosynthetic limitation and volatile and non-volatile isoprenoids in the poikilochlorophyllous resurrection plant Xerophyta humilis during dehydration and rehydration. Plant Cell Environ. 2012, 35, 2061–2074. [Google Scholar] [CrossRef]
- Tattini, M.; Velikova, V.; Vickers, C.; Brunetti, C.; di Ferdinando, M.; Trivellini, A.; Fineschi, S.; Agati, G.; Ferrini, F.; Loreto, F. Isoprene production in transgenic tobacco alters isoprenoid, non-structural carbohydrate and phenylpropanoid metabolism, and protects photosynthesis from drought stress. Plant Cell Environ. 2015, 37, 1950–1964. [Google Scholar] [CrossRef] [Green Version]
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action of volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291. [Google Scholar] [CrossRef]
- Velikova, V.; Tsonev, T.; Loreto, F.; Centritto, M. Changes in photosynthesis, mesophyll conductance to CO2, and isoprenoid emissions in Populus nigra plants exposed to excess nickel. Environ. Pollut. 2011, 159, 1058–1066. [Google Scholar] [CrossRef]
- Loreto, F.; Dicke, M.; Schnitzler, J.P.; Turlings, T.C.J. Plant volatiles and the environment. Plant Cell Environ. 2014, 37, 1905–1908. [Google Scholar] [CrossRef]
- Pegoraro, E.; Rey, A.; Greenberg, J.; Harley, P.; Grace, J.; Malhi, Y.; Guenther, A. Effect of drought on isoprene emission rates from leaves of Quercus virginiana Mill. Atmos. Environ. 2004, 38, 6149–6156. [Google Scholar] [CrossRef] [Green Version]
- Behnke, K.; Ghirardo, A.; Janz, D.; Kanawati, B.; Esperschütz, J.; Zimmer, I.; Schmitt-Kopplin, P.; Niinemets, U.; Polle, A.; Schnitzler, J.P.; et al. Isoprene function in two contrasting poplars under salt and sunflecks. Tree Physiol. 2013, 33, 562–578. [Google Scholar] [CrossRef] [Green Version]
- Junker, L.V.; Kleiber, A.; Jansen, K.; Wildhagen, H.; Hess, M.; Kayler, Z.; Kammerer, B.; Schnitzler, J.P.; Kreuzwieser, J.; Gessler, A.; et al. Variation in short-term and long-term responses of photosynthesis and isoprenoid-mediated photoprotection to soil water availability in four Douglas-fir provenances. Sci. Rep. 2017, 7, 40145. [Google Scholar] [CrossRef] [Green Version]






| Days After Salt Stress | Days After Water Stress | Photosynthesis | Stomatal Conductance | Isoprene Emission | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Salt Stress | Drought Stress | Salt X Drought Stress | Salt Stress | Drought Stress | Salt X Drought Stress | Salt Stress | Drought Stress | Salt X Drought Stress | ||
| 12 | - | ns | - | - | * | - | - | ns | - | - |
| 21 | - | * | - | - | ** | - | - | ns | - | - |
| 33 | - | *** | - | - | *** | - | - | *** | - | - |
| 46 | - | * | - | - | * | - | - | *** | - | - |
| 56 | - | ** | - | - | ** | - | - | ** | - | - |
| 63 | 2 | 83.3 *** | 11.9 ns | 4.8 ns | 58.5 ** | 34.0 ns | 7.5 ns | 76.8 *** | 17.2 ** | 6.0 * |
| 67 | 6 | 26.6 ** | 59.2 ** | 14.6* | 14.8 ns | 72.9 * | 12.3 ns | 50.7 *** | 25.9 * | 23.4 ** |
| 70 | 9 | 45.4 ** | 46.8 * | 7.8* | 32.0 * | 60.7 * | 7.3 * | 76.9 *** | 16.9 ** | 6.2 ns |
| 73 | 12 | 19.2 *** | 73.4 *** | 7.4 *** | 9.2 * | 88.0 *** | 72.8 | 66.0 * | 20.9 ns | 13.1 ns |
| 83 | 79.7 *** | 13.8 * | 6.0 * | 72.1 *** | 17.5 ** | 10.4 ** | 88.9 *** | 10.8 ns | 0.3 ns | |
| 136 | ns | ns | ns | ns | ns | ns | 50.0 ** | 32.4 ns | 17.6 * | |
| Days After Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 21 | 33 | 46 | 56 | 63(2) | 67(6) | 70(9) | 73(12) | 83 Drought Recovery | 136 Salt Recovery | |
| Fo | |||||||||||
| Control | 205.2 a | 195.5 a | 198.7 b | 196.0 b | 206.3 b | 192.1 b | 193.2 b | 195.9 b | 187.7 c | 211.0 b | 196.41 a |
| (±3.8) | (±2.7) | (±4.2) | (±3.3) | (±4.5) | (±3.9) | (±2.6) | (±3.8) | (±2.4) | (±7.3) | (±3.4) | |
| Drought | n.d. | n.d. | n.d. | n.d. | n.d. | 195.1 b | 196.6 b | 212.6 b | 233.4 b | 267.3 ab | 202.2 a |
| (±4.3) | (±2.9) | (±6.5) | (±9.4) | (±9.7) | (±9.1) | ||||||
| Salt | 190.4 b | 193.4 a | 251.3 a | 248.2 a | 237.5 a | 246.6 a | 258.3 a | 256.5 a | 271.3 ab | 250.7 ab | 182.6 a |
| (±3.6) | (±4.5) | (±21.3) | (±15.6) | (±16.4) | (±10,1) | (±14.4) | (±11.9) | (±78) | (±18.8) | (±6.8) | |
| Salt + drought | n.d. | n.d. | n.d. | n.d. | n.d. | 250.3 a | 246.9 a | 263.3 a | 283.3 a | 302.7 a | 194.5 a |
| (±17.5) | (±14.4) | (±18.0) | (±14.2) | (±30.7) | (±8.0) | ||||||
| Fv/Fm | |||||||||||
| Control | 0.79 a | 0.80 a | 0.80 a | 0.82 a | 0.81 a | 0.82 a | 0.84 a | 0.81 a | 0.82 a | 0.80 a | 0.79 a |
| (±0.01) | (±0.00) | (±0.01) | (±0.00) | (±0.00) | (±0.00) | (±0.00) | (±0.00) | (±0.00) | (±0.01) | (±0.01) | |
| Drought | n.d. | n.d. | n.d. | n.d. | n.d. | 0.81 a | 0.83 a | 0.79 a | 0.78 ab | 0.76 ab | 0.80 a |
| (±0.01) | (±0.00) | (±0.01) | (±0.01) | (±0.02) | (±0.01) | ||||||
| Salt | 0.80 a | 0.80 a | 0.76 b | 0.77 b | 0.77 b | 0.74 b | 0.72 b | 0.71 b | 0.71 bc | 0.69 ab | 0.79 a |
| (±0.01) | (±0.01) | (±0.01) | (±0.02) | (±0.01) | (±0.01) | (±0.03) | (±0.03) | (±0.02) | (±0.03) | (±0.01) | |
| Salt + drought | n.d. | n.d. | n.d. | n.d. | n.d. | 0.76 b | 0.75 ab | 0.68 b | 0.67 c | 0.68 c | 0.78 a |
| (±0.01) | (±0.02) | (±0.03) | (±0.03) | (±0.04) | (±0.00) | ||||||
| ΦPSII | |||||||||||
| Control | 0.19 a | 0.20 a | 0.16 a | 0.18 a | 0.14 a | 0.14 a | 0.14 a | 0.18 a | 0.13 a | 0.18 a | 0.18 a |
| (±0.01) | (±0.01) | (±0.03) | (±0.02) | (±0.01) | (±0.01) | (±0.01) | (±0.02) | (±0.01) | (±0.01) | (±0.03) | |
| Drought | n.d. | n.d. | n.d. | n.d. | n.d. | 0.12 ab | 0.11 a | 0.10 b | 0.04 b | 0.13 b | 0.16 a |
| (±0.02) | (±0.01) | (±0.01) | (±0.01) | (±0.01) | (±0.03) | ||||||
| Salt | 0.17 a | 0.12 b | 0.06 b | 0.07 b | 0.06 b | 0.07 bc | 0.06 b | 0.03 c | 0.07 b | 0.10 bc | 0.20 a |
| (±0.01) | (±0.01) | (±0.01) | (±0.01) | (±0.01) | (±0.01) | (±0.01) | (±0.01) | (±0.02) | (±0.02) | (±0.05) | |
| Salt + drought | n.d. | n.d. | n.d. | n.d. | n.d. | 0.06 c | 0.07 b | 0.05 c | 0.04 b | 0.06 c | 0.16 a |
| (±0.01) | (±0.01) | (±0.01) | (±0.01) | (±0.02) | (±0.02) | ||||||
| NPQ | |||||||||||
| Control | 2.44 a | 2.06 b | 2.34 b | 2.73 b | 1.72 b | 1.76 b | 2.34 c | 2.01 b | 0.57 b | 1.29 b | 2.10 a |
| (±0.26) | (±0.16) | (±0.39) | (±0.27) | (±0.19) | (±0.29) | (±0.45) | (±0.28) | (±0.10) | (±0.53) | (±0.37) | |
| Drought | n.d. | n.d. | n.d. | n.d. | n.d. | 1.86 b | 3.49 bc | 3.41 ab | 2.00 a | 1.62 b | 1.66 a |
| (±0.32) | (±0.14) | (±0.22) | (±0.26) | (±0.33) | (±0.28) | ||||||
| Salt | 2.97 a | 2.96 a | 3.68 a | 4.36 a | 3.87 a | 3.47 a | 4.65 ab | 4.50 a | 1.88 a | 2.81 ab | 2.18 a |
| (±0.36) | (±0.19) | (±0.45) | (±0.35) | (±0.30) | (±0.46) | (±0.47) | (±0.82) | (±0.17) | (±0.61) | (±0.35) | |
| Salt + drought | n.d. | n.d. | n.d. | n.d. | n.d. | 4.53 a | 5.06 a | 3.94 a | 2.30 a | 3.74 a | 2.48 a |
| (±0.33) | (±0.25) | (±0.37) | (±0.26) | (±0.43) | (±0.22) | ||||||
| Days After Salt Stress | Days After Water Stress | F0 | Fv/Fm | ΦPSII | NPQ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salt Stress | Drought Stress | Salt + Drought Stress | Salt Stress | Drought Stress | Salt + Drought Stress | Salt Stress | Drought Stress | Salt + Drought Stress | Salt Stress | Drought Stress | Salt + Drought Stress | ||
| 12 | - | * | - | - | ns | - | - | ns | - | - | ns | - | - |
| 21 | - | ns | - | - | ns | - | - | * | - | - | * | - | - |
| 33 | - | * | - | - | * | - | - | ** | - | - | * | - | - |
| 46 | - | ** | - | - | * | - | - | ** | - | - | * | - | - |
| 56 | - | * | - | - | ** | - | - | *** | - | * | *** | - | - |
| 63 | 2 | 78.3 ** | 20.6 ns | 1.1 ns | 76.2 *** | 18.7 ns | 5.1 ns | 61.9 ** | 27.5 ns | 10.6 ns | 60.8 ** | 37.8 ns | 1.4 ns |
| 67 | 6 | 83.1 ** | 11.9 ns | 5.0 ns | 57.9 *** | 26.4 * | 15.7 * | 67.9 *** | 13.0 ns | 19.1 * | 53.7 *** | 39.4 ** | 6.9 ns |
| 70 | 9 | 71.8 ** | 22.2 ns | 6.0 ns | 66.9 *** | 24.7 ** | 8.4 ns | 65.1 *** | 15.3 * | 19.6 *** | 48.0 ** | 28.4 ns | 23.6 * |
| 73 | 12 | 63.2 *** | 28.4 * | 8.4 ns | 72.1 *** | 25.8 ns | 2.1 ns | 9.7 * | 72.6 *** | 17.7 ** | 27.6 ** | 62.5 *** | 9.9 ns |
| 83 | 35.5 ns | 62.7 ns | 1.8 ns | ns | ns | ns | 57.8 ** | 41.2 ns | 1.0 ns | 68.2 *** | 30.6 ns | 1.2 ns | |
| 136 | ** | ns | ns | * | ns | ns | ns | ns | ns | * | ns | ns | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baraldi, R.; Przybysz, A.; Facini, O.; Pierdonà, L.; Carriero, G.; Bertazza, G.; Neri, L. Impact of Drought and Salinity on Sweetgum Tree (Liquidambar styraciflua L.): Understanding Tree Ecophysiological Responses in the Urban Context. Forests 2019, 10, 1032. https://doi.org/10.3390/f10111032
Baraldi R, Przybysz A, Facini O, Pierdonà L, Carriero G, Bertazza G, Neri L. Impact of Drought and Salinity on Sweetgum Tree (Liquidambar styraciflua L.): Understanding Tree Ecophysiological Responses in the Urban Context. Forests. 2019; 10(11):1032. https://doi.org/10.3390/f10111032
Chicago/Turabian StyleBaraldi, Rita, Arkadiusz Przybysz, Osvaldo Facini, Lorenzo Pierdonà, Giulia Carriero, Gianpaolo Bertazza, and Luisa Neri. 2019. "Impact of Drought and Salinity on Sweetgum Tree (Liquidambar styraciflua L.): Understanding Tree Ecophysiological Responses in the Urban Context" Forests 10, no. 11: 1032. https://doi.org/10.3390/f10111032
APA StyleBaraldi, R., Przybysz, A., Facini, O., Pierdonà, L., Carriero, G., Bertazza, G., & Neri, L. (2019). Impact of Drought and Salinity on Sweetgum Tree (Liquidambar styraciflua L.): Understanding Tree Ecophysiological Responses in the Urban Context. Forests, 10(11), 1032. https://doi.org/10.3390/f10111032