Impact of Rhabdocline pseudotsugae and Phaeocryptopus gaeumannii on the Selection of Suitable Provenances of Douglas Fir in Central Europe
Abstract
:1. Introduction
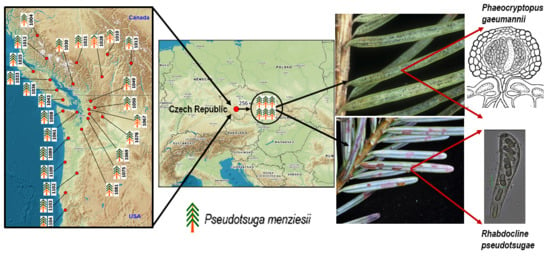
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kšír, J.; Beran, F.; Podrázský, V.; Novotný, P.; Dostál, J.; Kubeček, J. Výsledky hodnocení mezinárodní provenienční plochy s douglaskou tisolistou (Pseudotsuga menziesii /Mirb./Franco) na lokalitě Hůrky v jižních Čechách ve věku 44 let. Zpravy Les. Vyzk. 2015, 60, 104–114. [Google Scholar]
- Šindelář, J.; Beran, F. K některým aktuálním problémům pěstování douglasky tisolisté (orientační studie). Lesn. Pruvodce 2004, 3, 1–34. [Google Scholar]
- Kubeček, J.; Štefančík, I.; Podrázský, V.; Longauer, R. Výsledky výzkumu douglasky tisolisté (Pseudotsuga menziesii /Mirb./Franco) v České republice a na Slovensku—Přehled. Les. Cas. For. J. 2014, 60, 120–129. [Google Scholar] [CrossRef]
- Bastien, J.-C.; Sanchez, L.; Michaud, D. Douglas-Fir (Pseudotsuga menziesii (Mirb.) Franco). In Forest Tree Breeding in Europe, 1st ed.; Pâque, L., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 325–369. ISBN 978-94-007-6146-9. [Google Scholar]
- Podrázský, V.; Remeš, J. Půdotvorná role významných introdukovaných jehličnanů—Douglasky tisolisté, jedle obrovské a borovice vejmutovky. Zpravy Lesn. Vyzk. 2008, 53, 29–36. [Google Scholar]
- Podrázský, V.; Kubeček, J. Může douglaska tisolistá nahradit chřadnoucí smrk? Lesn. Prace 2014, 93, 14–19. [Google Scholar]
- Broncano, M.J.; Vila, M.; Boada, M. Evidence of Pseudotsuga menziesii naturalization in montane Mediterranean forests. For. Ecol. Manag. 2005, 211, 257–263. [Google Scholar] [CrossRef]
- Bušina, F. Natural regeneration of Douglas fir (Pseudotsuga menziesii [Mirb.] Franco) in forest stands of Training Forest District Hůrky, Higher Forestry School and Secondary Forestry School in Písek. J. For. Sci. 2007, 53, 20–34. [Google Scholar] [CrossRef]
- Hart, V.; Hartová, M.; Tauchman, P. Analysis of herbicide effects on Douglas fir (Pseudotsuga menziesii [Mirb.] Franco) natural regeneration. J. For. Sci. 2010, 56, 209–217. [Google Scholar] [CrossRef]
- Schmid, M.; Pautasso, M.; Holdenrieder, O. Ecological consequences of Douglas fir (Pseudotsuga menziesii) cultivation in Europe. Eur. J. For. Res. 2014, 133, 13–29. [Google Scholar] [CrossRef]
- Eberhard, B.; Hasenauer, H. Modeling Regeneration of Douglas fir forests in Central Europe. Austrian J. For. Sci. 2018, 135, 33–51. [Google Scholar]
- Cafourek, J. Dovoz osiva douglasky tisolisté do ČR. Lesn. Prace 2014, 93, 432–434. [Google Scholar]
- Martiník, A.; Houšková, K.; Palátová, E.; Cafourek, J.; Mauer, O. Předosevní Příprava a Doba Výsevu Douglasky Tisolisté (Pseudotsuga menziesii /Mirb./Franco), 1st ed.; Mendelova univerzita v Brně: Brno, Czech Republic, 2014; 16p, ISBN 978-80-7509-161-1. [Google Scholar]
- Šika, A. Reprodukční materiál douglasky tisolisté v ČSR z domácích zdrojů. Prace VULHM 1985, 67, 41–62. [Google Scholar]
- Jarry, M.; Candau, J.N.; Roques, A.; Ycart, B. Impact of emigrating seed chalcid, Megastigmus spermotrophus Wachtl (Hymenoptera: Torymidae), on seed production in a Douglas-fir seed orchard in France and modelling of orchard invasion. Can. Entomol. 1997, 129, 7–19. [Google Scholar] [CrossRef]
- Wolf, H. First results related to flushing and response to drought stress of Douglas fir (Pseudotsuga menziesii [Mirb.] Franco) progenies descending from Germany in comparison to provenances descending from North-America. Forstarchiv 2012, 83, 75–84. [Google Scholar]
- Farjon, A. A Handbook of the World’s Conifers, 1st ed.; Brill: Leiden, The Netherlands; Boston, MA, USA, 2010; Volume II, pp. 529–1111. ISBN 978-90-04-17718-5. [Google Scholar]
- Farjon, A.; Filer, D. An Atlas of the World’s Conifers: An Analysis of Their Distribution, Biogeography, Diversity and Conservation Status, 1st ed.; Brill: Leiden, The Netherlands; Boston, MA, USA, 2013; 512p, ISBN 978-90-04-21180-3. [Google Scholar]
- Lavender, D.P.; Hermann, R.K. Douglas-Fir. The Genus Pseudotsuga, 1st ed.; Oregon State University, College of Forestry, Forest Research Laboratory: Corvallis, OR, USA, 2014; 352p, ISBN 978-0-615-97995-3. [Google Scholar]
- Šika, A. Present results of the international provenance experiment of IUFRO with Douglas fir in the ČSR. Commun. Inst. For. Czech. 1981, 12, 83–101. [Google Scholar]
- Jenkins, M.J.; Hebertson, E.; Page, W.; Jorgensen, C.A. Bark beetles, fuels, fires and implications for forest management in the Intermountain West. For. Ecol. Manag. 2008, 254, 16–34. [Google Scholar] [CrossRef]
- Bansal, S.; Harrington, C.A.; St. Clair, J.B. Tolerance to multiple climate stressors: A case study of Douglas-fir drought and cold hardiness. Ecol. Evol. 2016, 6, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Ritóková, G.; Shaw, D.C.; Filip, G.; Kanaskie, A.; Browning, J.; Norlander, D. Swiss Needle Cast in Western Oregon Douglas-Fir Plantations: 20-Year Monitoring Results. Forests 2016, 7, 155. [Google Scholar] [CrossRef]
- Sakai, A.; Weiser, C.J. Freezing resistance of trees in North America with reference to tree regions. Ecology 1973, 54, 118–126. [Google Scholar] [CrossRef]
- Malmqvist, C.; Wallertz, K.; Johansson, U. Survival, early growth and impact of damage by late-spring frost and winter desiccation on Douglas-fir seedlings in southern Sweden. New For. 2018, 1–14. Available online: https://link.springer.com/article/10.1007/s11056-018-9635-7 (accessed on 20 January 2019). [CrossRef] [PubMed]
- Campbell, R.K.; Sorensen, F.C. Cold-acclimation in seedling Douglas fir related to phenology and provenance. Ecology 1973, 54, 1148–1151. [Google Scholar] [CrossRef]
- Boyce, J.S. A needle-cast of Douglas fir associated with Adelopus gäumanni. Phytopathology 1940, 30, 649–659. [Google Scholar]
- Hansen, E.M. Forest pathogens of N.W. North America and their potential for damage in Britain. For. Rec. UK For. Comm. 1985, 129, 1–14. [Google Scholar]
- Gernandt, D.S.; Camacho, F.H.; Stone, J.K. Meria laricis, an anamorph of Rhabdocline. Mycologia 1997, 89, 735–746. [Google Scholar] [CrossRef]
- Holah, C.J.; Wilson, V.M.; Hansen, M.E. Impacts of a native root-rotting pathogen on successional development of old-growth Douglas fir forests. Oecologia 1997, 111, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Hood, I.A.; Kimberley, M.O. Douglas fir provenance susceptibility to Swiss needle cast in New Zealand. Australas. Plant Pathol. 2005, 34, 57–62. [Google Scholar] [CrossRef]
- Kimberley, M.O.; Hood, I.A.; Knowles, R.L. Impact of Swiss needle-cast on growth of Douglas-fir. Phytopathology 2011, 101, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Łakomy, P.; Iwańczuk, M. Phaeocryptopus gaeumannii in Douglas-fir stands in Smolarz forest district. Phytopathologia 2010, 58, 43–52. [Google Scholar]
- Ogris, N.; Jurc, D. Tree diseases determined by the reporting, prognostic and diagnostic service for forests in Slovenia 1982–2012. Acta Silvae Ligni 2013, 102, 31–42. [Google Scholar] [CrossRef]
- Diminić, D.; Gršković, M.; Mozer, A. Fungual diseases of coniferous trees in plantations in North Adriatic area of Croatia. Glas. Sum. Pokuse 2016, 5, 355–364. [Google Scholar]
- Spiecker, H.; Lindner, M.; Schuler, J. (Eds.) Douglas-Fir—An Option for Europe; EFI What Science Can Tell Us 9; European Forest Institute: Joensuu, Finland, 2019; 121p, ISBN 978-952-5980-66-0. [Google Scholar]
- Hansen, E.M.; Stone, J.K.; Capitano, B.R.; Rosso, P.; Sutton, W.; Winton, L. Incidence and Impact of Swiss Needle Cast in Forest Plantations of Douglas-fir in Coastal Oregon. Plant Dis. 2000, 84, 773–778. [Google Scholar] [CrossRef]
- Weir, J.R. A needle blight of the Douglas fir. J. Agric. Res. 1917, 10, 99–105. [Google Scholar]
- Wilson, M.; Wilson, M.J.F. Rhabdocline pseudotsugae Syd.: A new disease of the Douglas fir in Scotland. Trans. R. Scot. Arbor. Soc. 1926, 40, 37–40. [Google Scholar]
- Brown, A.B. Observation on leaf fall in the Douglas fir when infected with Rhabdocline pseudotsugae Sydow. Ann. Appl. Biol. 1930, 17, 745–754. [Google Scholar] [CrossRef]
- Kalandra, A. První výskyt sypavky duglasky—Působené houbou Rhabdocline pseudotsugae Syd.—V odstoupeném Sudetském území Čech. Ochrana Rostlin 1939, 15, 36–40. [Google Scholar]
- Morgenstern, K.; Polster, J.-U.; Krabel, D. Genetic variation between and within two populations of Rhabdocline pseudotsugae in Germany. Can. J. For. Res. 2016, 46, 716–724. [Google Scholar] [CrossRef]
- Butin, H. Tree Diseases and Disorders. Causes, Biology and Control in Forest and Amenity Trees, 1st ed.; Oxford University Press: New York, NY, USA; Tokyo, Japan, 1995; 252p, ISBN 0198549326. [Google Scholar]
- Van Vloten, H. Rhabdocline Pseudotsugae Sydow: Oorzaak Eener Ziekte van Douglasspar. Ph.D. Thesis, Mededeling/Instituut voor Phytopathologie, Laboratorium voor Mycologie en Aardappelonderzoek, Wageningen, The Netherlands, 1932; 171p. [Google Scholar]
- Gäumann, E. Über eine neue Krankheit der Douglasien. Z. Pflanzenkrankh. Pflanzenschutz 1930, 40, 305–313. [Google Scholar]
- Michaels, E.; Chastagner, G.A. Distribution, severity, and impact of Swiss needle cast in Douglas-fir Christmas trees in western Washington and Oregon. Plant Dis. 1984, 68, 939–942. [Google Scholar] [CrossRef]
- Temel, F.; Johnson, G.R.; Stone, J.K. The relationship between Swiss needle cast symptom severity and level of Phaeocryptopus gaeumannii colonization in coastal Douglas-fir (Pseudotsuga menziesii var. menziesii). For. Pathol. 2004, 34, 383–394. [Google Scholar] [CrossRef]
- Stone, J.K.; Capitano, B.R.; Kerrigan, J.L. The histopathology of Phaeocryptopus gaeumannii on Douglas-fir needles. Mycologia 2008, 100, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Petkova, K.; Georgieva, M.; Uzunov, M. Investigation of Douglas-fir provenance test in North-Western Bulgaria at the age of 24 years. J. For. Sci. 2014, 60, 288–296. [Google Scholar] [CrossRef]
- Pešková, V. Rhabdocline pseudotsugae Sydow. Skotská sypavka douglasky. Lesn. Prace 2003, 82. Available online: http://lmda.silvarium.cz/search/i.jsp?pid=uuid:17f63f3c-1e0e-47a3-90ed-6c99df079585#periodical-periodicalvolume-periodicalitem-supplement-page_uuid:d76be9a1-c570-11e4-8912-001b63bd97ba (accessed on 10 March 2018).
- Hood, I.A. Menziesii in southern British Columbia. NZJ For. Sci. 1982, 12, 415–424. [Google Scholar]
- Hood, I.A.; Sandberg, C.J.; Barr, C.W.; Holloway, W.A.; Bradbury, P.M. Changes in needle retention associated with the spread an estab-lishment of Phaeocryptopus gaeumannii in planted Douglas-fir. Eur. J. For. Pathol. 1990, 20, 418–429. [Google Scholar] [CrossRef]
- Bergel, D. Die Herleitung neuer Massentafeln für die Douglasie in Nordwestdeutschland. Allg. Forst Jagdzeitung 1971, 142, 247–256. [Google Scholar]
- Konšel, J. Stručný Nástin Tvorby a Pěstění Lesů v Biologickém Ponětí, 1st ed.; Matice lesnická: Písek, Czechoslovakia, 1931; 552p. [Google Scholar]
- Bosshard, W. Sanasilva-Kronenbilder; Couronnes d’arbres; Le chiome degli alberi. In Eidgenijssische Anstalt filr das Forstiiche Versuchswesen; Eidgenössische Forschungsanstalt für Wald, Schnee und Landschaft: Birmensdorf, Switzerland, 1986. [Google Scholar]
- Pekár, S.; Brabec, M. Moderní Analýza Biologických Dat 1, 1st ed.; Scientia: Praha, Czech Republic, 2009; 225p, ISBN 978-80-86960-44-9. [Google Scholar]
- Crawley, M. The R Book, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2013; 1051p, ISBN 978-0-470-97392-9. [Google Scholar]
- Burger, S.V. Introduction to Machine Learning with R—Rigorous Mathematical Modelling; O’Reilly: Sebastopol, CA, USA, 2018; 226p, ISBN 978-1491976449. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 28 December 2018).
- Beran, F. Dosavadní výsledky provenienčního výzkumu douglasky tisolisté v ČR. Zpravy Les. Vyzk. 1995, 40, 7–13. [Google Scholar]
- Catal, M.; Adams, G.C.; Fulbright, D.W. Evaluation of Resistance to Rhabdocline Needlecast in Douglas Fir Variety Shuswap, with Quantitative Polymerase Chain Reaction. Phytopathology 2010, 100, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, K.; Döring, M.; Krabel, D. Rhabdocline needle cast—Investigations on various Douglas fir tissue types. Eur. J. Plant Pathol. 2013, 137, 495–504. [Google Scholar] [CrossRef]
- Eilmann, B.; de Vries, S.M.G.; den Ouden, J.; Mohren, G.M.J.; Sauren, P.; Sass-Klaassen, U. Origin matters! Difference in drought tolerance and productivity of coastal Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) provenances. For. Ecol. Manag. 2013, 302, 133–143. [Google Scholar] [CrossRef]
- Georgieva, M. Diseases on Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) and Their Influence on the Species Introduction in Bulgaria. Ph.D. Thesis, Forest Research Institute-BAS, Sofia, Bulgaria, 2009. (In Bulgarian). [Google Scholar]
- Petkova, K. Investigation of Douglas-fir provenance test in North-Western Bulgaria at age 20. For. Ideas 2011, 17, 1–42. [Google Scholar]
- Popov, E.B. Results of 20 years old Douglas-fir provenance experiment established on the northern slopes of Rila Mountain in Bulgaria. J. For. Sci. 2014, 60, 394–399. [Google Scholar] [CrossRef]
- Milenkova, A.; Konnert, M.; Fusi, B.; Petkova, K. Identification of varieties and genetic diversity of Douglas fir stands in the region of Osogovo, Southwest Bulgaria. For. Ideas 2018, 24, 37–50. [Google Scholar]
- Maguire, D.A.; Kanaskie, A.; Voelker, W.; Johnson, R.; Johnson, G. Growth of young Douglas-fir plantations across a gradient in Swiss needle cast severity. West. J. Appl. For. 2002, 17, 86–95. [Google Scholar] [CrossRef]
- Reyer, C.; Lasch, P.; Mohren, G.M.J.; Sterck, F. Inter-specific competition in mixed forests of Douglas-fir (Pseudotsuga menziesii) and common beech (Fagus sylvatica) under climate change—A model-based analysis. Ann. For. Sci. 2010, 67, 805. [Google Scholar] [CrossRef]
- Vitali, V.; Buntgen, U.; Bauhus, J. Seasonality matters—The effects of past and projected seasonal climate change on the growth of native and exotic conifer species in Central Europe. Dendrochronologia 2018, 48, 1–9. [Google Scholar] [CrossRef]




| State (USA), Province (Canada) | Provenance | Provenance Area | Elevation (m a.s.l.) | North Latitude | West Longitude | |
|---|---|---|---|---|---|---|
| British Columbia | 1004 | Stuie | Maritime | 230 | 52°22′ | 126°00′ |
| 1010 | Barrière | Cariboo Trans | 612 | 51°12′ | 120°10′ | |
| 1012 | Klina Klini | Maritime | 3 | 51°07′ | 125°36′ | |
| 1013 | Revelstoke | Nelson | 600 | 51°00′ | 118°12′ | |
| 1021 | D’Arcy | Submaritime | 275 | 50°33′ | 122°30′ | |
| 1025 | Nimkish | Maritime | 90 | 50°19′ | 126°53′ | |
| 1028 × | Merritt | Zone Not Defined | 870 | 50°04′ | 120°51′ | |
| 1030 | Squamish | Maritime | 15 | 49°47′ | 123°09′ | |
| 1033 | Forbidden | Maritime | 610 | 49°40′ | 125°09′ | |
| 1036 | Alberni | Maritime | 140 | 49°19′ | 124°51′ | |
| 1043 | San Juan | Maritime | 215 | 48°35′ | 124°05′ | |
| Washington | 1049 | Bacon Point | 7–Skagit | 500 | 48°36′ | 121°23′ |
| 1050 | Marblemount | 7–Skagit | 120 | 48°35′ | 121°24′ | |
| 1058 | Lake Crescent | 1–Hoh | 305 | 48°04′ | 124°00′ | |
| 1061 | Louella | 1–Hoh | 457 | 48°00′ | 123°05′ | |
| 1067 | Skykomish | 5–Kitsap | 305 | 47°42′ | 121°20′ | |
| 1069 | North Bend | 8–Snoqualmie | 150 | 47°28′ | 121°45′ | |
| 1075 | Enumclaw | 5–Kitsap | 240 | 47°16′ | 121°56′ | |
| 1078 × | Cle Elum | 8–Snoqualmie | 640 | 47°13′ | 121°07′ | |
| 1081 × | Alder Lake | 9–Toutle | 430 | 46°48′ | 122°17′ | |
| 1089 | Cathlamet | 3–Twin Harbors | 200 | 46°18′ | 123°16′ | |
| Oregon | 1100 | Grand Ronde | 6 | 200 | 45°06′ | 123°36′ |
| 1102 × | Upper Soda | 12 | 1000 | 44°23′ | 122°12′ | |
| 1103 | Coquille | 2 | 100 | 43°12′ | 124°10′ | |
| 1104 × | Brookings | 1 | 300 | 42°07′ | 124°12′ | |
| April | May | June | July | August | Total | |
|---|---|---|---|---|---|---|
| Rhabdocline pseudotsugae | 0 | 14 | 41 | 4 | 0 | 59 |
| Phaeocryptopus gaeumannii | 0 | 1 | 38 | 201 | 0 | 240 |
| State (USA), Province (Canada) | Provenance | Coastal (C)/Interior (I) | Number of trees | Height (m) | Breast-height diameter (cm) | Standing Volume of One Tree with Bark (m3) | Defoliation (%) | Number of Trees with 100% Defoliation | |
|---|---|---|---|---|---|---|---|---|---|
| British Columbia | 1004 | Stuie | C | 27 | 32 | 31.9 | 1.16 | 34 | 2 |
| 1010 | Barrière | I | 32 | 26 | 25.1 | 0.64 | 61 | 10 | |
| 1012 | Klina Klini | C | 27 | 31.3 | 31.4 | 1.12 | 31 | 1 | |
| 1013 | Revelstoke | I | 40 | 31.4 | 32.6 | 1.22 | 28 | 1 | |
| 1021 | D’Arcy | I | 24 | 28.7 | 29.8 | 0.95 | 48 | 3 | |
| 1025 | Nimkish | C | 30 | 30.6 | 29.2 | 0.99 | 32 | 2 | |
| 1028 × | Merritt | I | 15 | 18.3 | 15.9 | 0.21 | 90 | 14 | |
| 1030 | Squamish | C | 36 | 32.2 | 30.5 | 1.2 | 36 | 3 | |
| 1033 | Forbidden | C | 38 | 30.5 | 29.5 | 1.01 | 36 | 5 | |
| 1036 | Alberni | C | 27 | 32.3 | 34 | 1.36 | 34 | 1 | |
| 1043 | San Juan | C | 32 | 29.9 | 29.6 | 1.02 | 38 | 3 | |
| Washington | 1049 | Bacon Point | C | 31 | 30.6 | 30.9 | 1.1 | 33 | 1 |
| 1050 | Marblemount | C | 31 | 32.2 | 32.1 | 1.18 | 30 | 1 | |
| 1058 | Lake Crescent | C | 31 | 32.1 | 32.7 | 1.26 | 28 | 0 | |
| 1061 | Louella | C | 21 | 30.6 | 33.5 | 1.35 | 37 | 3 | |
| 1067 | Skykomish | C | 45 | 25.2 | 22.4 | 0.48 | 53 | 7 | |
| 1069 | North Bend | C | 29 | 32.7 | 35.3 | 1.42 | 23 | 0 | |
| 1075 | Enumclaw | C | 30 | 33.5 | 37.7 | 1.72 | 27 | 1 | |
| 1078 × | Cle Elum | I | 28 | 25.9 | 25.7 | 0.70 | 46 | 5 | |
| 1081 × | Alder Lake | C | 18 | 31.5 | 32.8 | 1.29 | 31 | 0 | |
| 1089 | Cathlamet | C | 31 | 31.9 | 33.5 | 1.27 | 24 | 0 | |
| Oregon | 1100 | Grand Ronde | C | 26 | 30.8 | 33.0 | 1.21 | 34 | 3 |
| 1102 × | Upper Soda | C | 31 | 28.8 | 30.4 | 1.07 | 39 | 4 | |
| 1103 | Coquille | C | 19 | 32.4 | 31.8 | 1.22 | 37 | 1 | |
| 1104 × | Brookings | C | 5 | 29.5 | 31.5 | 1.01 | 26 | 0 | |
| ∑/Average | 704 | 30.1 | 30.5 | 1.09 | 37 | 71 | |||
| Df | Deviance | Residual Df | Resid. Deviance | F | Pr (>F) | ||
|---|---|---|---|---|---|---|---|
| NULL | 558 | 86.412 | |||||
| volume | 1 | 42.627 | 557 | 43.785 | 728.487 | 2.20 × 10−16 | *** |
| provenance | 22 | 4.908 | 535 | 38.878 | 3.8122 | 2.23 × 10−8 | *** |
| diameter difference | 1 | 3.933 | 534 | 34.945 | 67.2096 | 1.98 × 10−15 | *** |
| volume:provenance | 22 | 2.903 | 512 | 32.042 | 2.2552 | 0.001 | *** |
| volume: diameter difference | 1 | 1.337 | 511 | 30.705 | 22.8495 | 2.29 × 10−6 | *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samek, M.; Novotný, P.; Modlinger, R.; Fulín, M.; Beran, F.; Roy, A.; Pešková, V. Impact of Rhabdocline pseudotsugae and Phaeocryptopus gaeumannii on the Selection of Suitable Provenances of Douglas Fir in Central Europe. Forests 2019, 10, 204. https://doi.org/10.3390/f10030204
Samek M, Novotný P, Modlinger R, Fulín M, Beran F, Roy A, Pešková V. Impact of Rhabdocline pseudotsugae and Phaeocryptopus gaeumannii on the Selection of Suitable Provenances of Douglas Fir in Central Europe. Forests. 2019; 10(3):204. https://doi.org/10.3390/f10030204
Chicago/Turabian StyleSamek, Michal, Petr Novotný, Roman Modlinger, Martin Fulín, František Beran, Amit Roy, and Vítězslava Pešková. 2019. "Impact of Rhabdocline pseudotsugae and Phaeocryptopus gaeumannii on the Selection of Suitable Provenances of Douglas Fir in Central Europe" Forests 10, no. 3: 204. https://doi.org/10.3390/f10030204
APA StyleSamek, M., Novotný, P., Modlinger, R., Fulín, M., Beran, F., Roy, A., & Pešková, V. (2019). Impact of Rhabdocline pseudotsugae and Phaeocryptopus gaeumannii on the Selection of Suitable Provenances of Douglas Fir in Central Europe. Forests, 10(3), 204. https://doi.org/10.3390/f10030204