Light Shock Stress after Outdoor Sunlight Exposure in Seedlings of Picea abies (L.) Karst. and Pinus sylvestris L. Pre-Cultivated under LEDs—Possible Mitigation Treatments and Their Energy Consumption
Abstract
:1. Introduction
- Lack of protective mechanisms against high light intensity. Light intensity levels during indoor cultivation are usually much lower than intensity levels outdoors. Hence, seedlings could be exposed to higher light intensity during parts of the indoor cultivation and perhaps also need to be transferred to outdoor conditions through an adaptation phase under shade cloths [56,57,58].
2. Materials and Methods
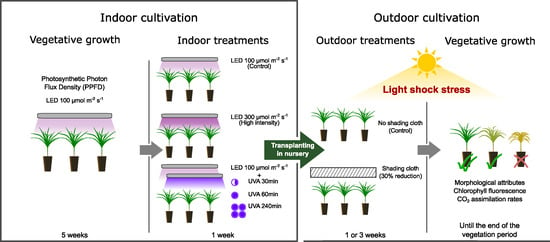
2.1. Plant Material
2.2. Indoor Cultivation
- One week for germination,
- Four weeks of vegetative growth,
- One week for indoor treatments.
2.2.1. Indoor Treatments
2.2.2. Light Sources
2.3. Outdoor Cultivation
2.3.1. Outdoor Treatments
2.3.2. Solar Radiation and Ambient Temperature
2.4. Seedling Measurements
2.4.1. Chlorophyll Fluorescence
2.4.2. Photosynthetic Light-Response Curves
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
- The main objective of the study, which was to identify successful protocols for light shock mitigation, was not reached. The applied treatments did not significantly improve the adaptation of the tree seedlings cultivated only under LED lights when transplanted to outdoor conditions. Nevertheless, there was a tendency that some preconditioning could reduce the light shock during the first days of transplanting, especially the high light intensity and the shading cloth treatments. Therefore, there is a need for additional studies with a broader approach of treatments that could mitigate the light shock stress.
- Both the spectral characteristics as well as the light intensity in the growth room facilities differ considerably from the natural outdoor conditions as seen in Figure 1. Further studies on the topic should focus on finding indoor treatments that adjust gradually the light intensity and spectrum including UV-light to levels that are closer to the outdoor conditions.
- Regardless of whether the seedlings had been treated or not, the chlorophyll fluorescence levels revealed signs of light shock stress and damage of the PSII after transplanting to direct sunlight. The most affected seedlings were those in the control group, especially Norway spruce, with a significant decrease in their maximum quantum yield of the PSII.
- Gas exchange measurements showed the acclimation of the seedlings from a more comprehensive perspective (not only the photoreceptor but the whole photosynthetic apparatus) and revealed a change in the light saturation points of the photosynthetic light-response curves of seedlings of both species after 35 days outdoors. This shift towards higher PPFD evidence an adaptation of the photosynthetic apparatus that allows the seedlings to cope with elevated light intensities and sustain higher CO2 uptake and faster growth in outdoor conditions.
- At the end of the vegetation period, seedlings from all treatments were able to withstand and recover from the light shock stress. The morphological attributes showed no differences between the treatments within each sowing (height:diameter and shoot:root ratio). Nevertheless, since seedlings of all treatments were affected, it is difficult to predict the full development potential if the light shock stress had been avoided.
- The properties of the indoor treatments (light intensity level or duration of the UV exposure) had a major impact on the energy consumption. Therefore, these factors should be further analyzed in future studies to continue developing the concept of year-round cultivation of forest seedlings in the boreal climate region.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Norway Spruce | Sowing 1 (2014-05-05) | Sowing 2 (2014-06-17) | Sowing 3 (2015-05-25) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoor Treatments | Indoor Treatments | Indoor Treatments | |||||||||||||||||||||
| Attribute | Outdoor Treatments | Control | High Intensity | UVA 30 min | UVA 60 min | Control | High Intensity | UVA 30 min | UVA 60 min | Control | High Intensity | UVA 240 min | |||||||||||
| Shoot height (mm) | No shading cloth (control) | 75.4 | (3.7) | 82.6 | (4.0) | 63.4 | (3.0) | 75.2 | (2.7) | 40.8 | (1.5) | 40.0 | (1.2) | 41.4 | (2.5) | 45.8 | (0.7) | 61.1 | (2.5) | 62.1 | (1.5) | 64.9 | (2.0) |
| Shading cloth-1 week | 90.6 | (2.3) | 87.2 | (4.5) | 89.0 | (3.0) | 92.8 | (3.8) | 45.6 | (2.1) | 45.0 | (1.9) | 45.6 | (0.5) | 41.6 | (1.0) | 61.3 | (1.4) | |||||
| Shading cloth-3 weeks | 94.0 | (2.2) | 94.6 | (4.7) | 86.2 | (4.0) | 90.4 | (2.3) | 44.6 | (1.2) | 42.4 | (1.7) | 45.8 | (2.2) | 45.8 | (1.8) | |||||||
| Stem diameter (mm) | No shading cloth (control) | 0.94 | (0.05) | 1.06 | (0.09) | 0.88 | (0.07) | 0.98 | (0.04) | 0.48 | (0.02) | 0.54 | (0.02) | 0.56 | (0.05) | 0.52 | (0.04) | 0.88 | (0.03) | 0.92 | (0.02) | 0.92 | (0.02) |
| Shading cloth-1 week | 1.18 | (0.06) | 1.14 | (0.06) | 1.12 | (0.05) | 1.26 | (0.07) | 0.58 | (0.04) | 0.54 | (0.02) | 0.54 | (0.05) | 0.70 | (0.03) | 0.89 | (0.03) | |||||
| Shading cloth-3 weeks | 1.14 | (0.12) | 1.08 | (0.04) | 1.08 | (0.04) | 1.12 | (0.05) | 0.64 | (0.05) | 0.58 | (0.04) | 0.60 | (0.03) | 0.60 | (0.03) | |||||||
| Height: diameter | No shading cloth (control) | 81.0 | (5.2) | 79.0 | (3.4) | 73.0 | (3.2) | 77.1 | (3.9) | 85.2 | (2.1) | 74.6 | (3.5) | 76.1 | (6.9) | 89.8 | (6.3) | 69.3 | (2.4) | 67.9 | (1.6) | 70.7 | (2.0) |
| Shading cloth-1 week | 77.3 | (2.8) | 76.8 | (3.5) | 79.9 | (3.3) | 74.1 | (2.4) | 80.1 | (7.1) | 83.7 | (3.9) | 87.3 | (7.7) | 59.9 | (2.9) | 69.3 | (2.0) | |||||
| Shading cloth-3 weeks | 85.8 | (7.9) | 87.5 | (2.1) | 79.9 | (2.6) | 81.5 | (4.7) | 71.3 | (5.2) | 74.6 | (6.3) | 77.7 | (7.3) | 77.7 | (6.5) | |||||||
| Shoot DW (g) | No shading cloth (control) | 0.163 | (0.015) | 0.172 | (0.064) | 0.099 | (0.031) | 0.144 | (0.023) | 0.030 | (0.007) | 0.028 | (0.003) | 0.031 | (0.006) | 0.032 | (0.003) | 0.113 | (0.034) | 0.121 | (0.021) | 0.127 | (0.022) |
| Shading cloth-1 week | 0.207 | (0.019) | 0.201 | (0.013) | 0.217 | (0.044) | 0.235 | (0.059) | 0.039 | (0.008) | 0.037 | (0.005) | 0.037 | (0.004) | 0.033 | (0.004) | 0.112 | (0.026) | |||||
| Shading cloth-3 weeks | 0.260 | (0.014) | 0.219 | (0.017) | 0.219 | (0.033) | 0.227 | (0.039) | 0.040 | (0.002) | 0.033 | (0.006) | 0.034 | (0.002) | 0.033 | (0.005) | |||||||
| Root DW (g) | No shading cloth (control) | 0.037 | (0.003) | 0.038 | (0.006) | 0.027 | (0.006) | 0.036 | (0.002) | 0.007 | (0.001) | 0.008 | (0.001) | 0.009 | (0.001) | 0.009 | (0.001) | 0.031 | (0.002) | 0.039 | (0.002) | 0.039 | (0.003) |
| Shading cloth-1 week | 0.057 | (0.008) | 0.053 | (0.008) | 0.054 | (0.003) | 0.053 | (0.006) | 0.010 | (0.001) | 0.011 | (0.001) | 0.010 | (0.001) | 0.009 | (0.001) | 0.036 | (0.004) | |||||
| Shading cloth-3 weeks | 0.072 | (0.008) | 0.058 | (0.011) | 0.048 | (0.005) | 0.056 | (0.003) | 0.011 | (0.001) | 0.008 | (0.001) | 0.008 | (0.001) | 0.008 | (0.001) | |||||||
| Shoot: root | No shading cloth (control) | 4.69 | (0.78) | 4.60 | (0.34) | 4.12 | (0.51) | 4.01 | (0.16) | 4.41 | (0.39) | 3.45 | (0.41) | 3.33 | (0.17) | 3.67 | (0.35) | 3.68 | (0.15) | 3.15 | (0.14) | 3.36 | (0.19) |
| Shading cloth-1 week | 3.77 | (0.35) | 3.89 | (0.29) | 4.00 | (0.18) | 4.49 | (0.20) | 3.75 | (0.21) | 3.66 | (0.50) | 3.57 | (0.27) | 3.72 | (0.48) | 3.24 | (0.16) | |||||
| Shading cloth-3 weeks | 3.82 | (0.54) | 4.24 | (0.77) | 4.76 | (0.44) | 4.12 | (0.33) | 3.84 | (0.45) | 4.23 | (0.62) | 4.92 | (0.89) | 3.90 | (0.10) | |||||||
| Sample mean (SE), 12 weeks after transplanting, n = 5 | Sample mean (SE), 6 weeks after transplanting, n = 5 | Sample mean (SE), 14 weeks after transplanting, n = 15 | |||||||||||||||||||||
| Scots Pine | Sowing 1 (2014-05-05) | Sowing 2 (2014-06-17) | Sowing 3 (2015-05-25) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoor Treatments | Indoor Treatments | Indoor Treatments | |||||||||||||||||||||
| Attribute | Outdoor Treatments | Control | High Intensity | UVA 30 min | UVA 60 min | Control | High Intensity | UVA 30 min | UVA 60 min | Control | High Intensity | UVA 240 min | |||||||||||
| Shoot height (mm) | No shading cloth (control) | 94.4 | (5.1) | 87.8 | (4.6) | 99.4 | (5.1) | 93.8 | (3.7) | 49.6 | (2.1) | 56.8 | (2.5) | 52.0 | (1.7) | 56.2 | (2.6) | 65.4 | (1.7) | 63.9 | (1.5) | 69.1 | (1.4) |
| Shading cloth-1 week | 91.8 | (5.6) | 88.8 | (3.1) | 89.2 | (1.7) | 104.2 | (5.5) | 51.0 | (1.7) | 52.6 | (1.4) | 53.6 | (2.7) | 57.2 | (1.4) | 66.3 | (1.8) | |||||
| Shading cloth-3 weeks | 91.2 | (7.1) | 83.2 | (4.9) | 94.4 | (5.5) | 96.4 | (3.6) | 52.6 | (3.1) | 55.6 | (3.5) | 55.0 | (3.6) | 52.6 | (2.5) | |||||||
| Stem diameter (mm) | No shading cloth (control) | 1.70 | (0.08) | 1.80 | (0.10) | 1.66 | (0.07) | 1.62 | (0.08) | 0.64 | (0.04) | 0.70 | (0.08) | 0.52 | (0.05) | 0.66 | (0.06) | 1.25 | (0.04) | 1.26 | (0.02) | 1.30 | (0.03) |
| Shading cloth-1 week | 1.78 | (0.12) | 1.54 | (0.09) | 1.44 | (0.05) | 1.82 | (0.06) | 0.68 | (0.05) | 0.72 | (0.04) | 0.78 | (0.02) | 0.74 | (0.07) | 1.29 | (0.03) | |||||
| Shading cloth-3 weeks | 1.76 | (0.11) | 1.64 | (0.07) | 1.66 | (0.08) | 1.66 | (0.07) | 0.88 | (0.05) | 0.72 | (0.09) | 0.80 | (0.03) | 0.90 | (0.03) | |||||||
| Height: diameter | No shading cloth (control) | 55.9 | (3.2) | 49.6 | (4.4) | 59.9 | (1.9) | 58.4 | (3.3) | 78.2 | (3.3) | 87.6 | (15.0) | 103.8 | (11.2) | 87.5 | (7.9) | 52.5 | (1.6) | 51.0 | (1.3) | 53.3 | (1.2) |
| Shading cloth-1 week | 52.2 | (3.8) | 58.2 | (2.8) | 62.2 | (1.9) | 57.6 | (4.1) | 76.5 | (5.8) | 73.9 | (4.3) | 69.0 | (4.5) | 80.0 | (7.5) | 51.7 | (1.4) | |||||
| Shading cloth-3 weeks | 52.2 | (3.8) | 51.2 | (4.0) | 57.2 | (3.3) | 58.2 | (1.4) | 60.6 | (5.4) | 85.5 | (18.2) | 69.7 | (6.8) | 58.5 | (2.0) | |||||||
| Shoot DW (g) | No shading cloth (control) | 0.524 | (0.026) | 0.568 | (0.064) | 0.547 | (0.031) | 0.500 | (0.023) | 0.066 | (0.007) | 0.073 | (0.003) | 0.064 | (0.006) | 0.065 | (0.003) | 0.226 | (0.034) | 0.228 | (0.021) | 0.245 | (0.022) |
| Shading cloth-1 week | 0.581 | (0.038) | 0.518 | (0.013) | 0.473 | (0.044) | 0.634 | (0.059) | 0.072 | (0.008) | 0.072 | (0.005) | 0.076 | (0.004) | 0.069 | (0.004) | 0.230 | (0.026) | |||||
| Shading cloth-3 weeks | 0.580 | (0.071) | 0.529 | (0.017) | 0.553 | (0.033) | 0.542 | (0.039) | 0.081 | (0.002) | 0.090 | (0.006) | 0.088 | (0.002) | 0.083 | (0.005) | |||||||
| Root DW (g) | No shading cloth (control) | 0.185 | (0.030) | 0.203 | (0.030) | 0.149 | (0.015) | 0.147 | (0.015) | 0.022 | (0.003) | 0.020 | (0.004) | 0.021 | (0.003) | 0.019 | (0.004) | 0.095 | (0.006) | 0.104 | (0.005) | 0.106 | (0.006) |
| Shading cloth-1 week | 0.184 | (0.024) | 0.162 | (0.041) | 0.121 | (0.004) | 0.176 | (0.018) | 0.018 | (0.002) | 0.023 | (0.002) | 0.020 | (0.001) | 0.020 | (0.002) | 0.107 | (0.008) | |||||
| Shading cloth-3 weeks | 0.135 | (0.020) | 0.156 | (0.024) | 0.173 | (0.012) | 0.176 | (0.009) | 0.020 | (0.003) | 0.029 | (0.004) | 0.022 | (0.003) | 0.017 | (0.002) | |||||||
| Shoot: root | No shading cloth (control) | 3.20 | (0.65) | 2.95 | (0.32) | 3.76 | (0.31) | 3.51 | (0.41) | 3.13 | (0.43) | 4.03 | (0.57) | 3.14 | (0.16) | 4.03 | (0.69) | 2.44 | (0.11) | 2.22 | (0.08) | 2.35 | (0.07) |
| Shading cloth-1 week | 3.35 | (0.42) | 3.87 | (0.80) | 3.87 | (0.20) | 3.72 | (0.36) | 4.14 | (0.51) | 3.32 | (0.48) | 3.79 | (0.30) | 3.50 | (0.17) | 2.26 | (0.13) | |||||
| Shading cloth-3 weeks | 4.39 | (0.18) | 3.58 | (0.39) | 3.23 | (0.22) | 3.06 | (0.26) | 4.28 | (0.42) | 3.27 | (0.25) | 4.10 | (0.41) | 4.99 | (0.48) | |||||||
| Sample mean (SE), 12 weeks after transplanting, n = 5 | Sample mean (SE), 6 weeks after transplanting, n = 5 | Sample mean (SE), 14 weeks after transplanting, n = 15 | |||||||||||||||||||||
References
- Mattsson, A.; Radoglou, K.; Kostopoulou, P.; Bellarosa, R.; Simeone, M.C.; Schirone, B. Use of innovative technology for the production of high-quality forest regeneration materials. Scand. J. For. Res. 2010, 25, 3–9. [Google Scholar] [CrossRef]
- Astolfi, S.; Marianello, C.; Grego, S.; Bellarosa, R. Preliminary investigation of LED lighting as growth light for seedlings from different tree species in growth chambers. Not. Bot. Hort. Agrobot. Cluj-Napoca 2012, 40, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Landis, T.D.; Pinto, J.R.; Dumroese, R.K. Light emitting diodes (LED): Applications in forest and native plant nurseries. For. Nurs. Notes 2013, 33, 5–13. [Google Scholar]
- Apostol, K.G.; Dumroese, R.K.; Pinto, J.R.; Davis, A.S. Light-emitting diode lighting for forest nursery seedling production. In Proceedings of the 2014 Annual Meeting of the International Plant Propagators Society, Hickory, NC, USA, 27 October 2014; pp. 335–339. [Google Scholar]
- Riikonen, J.; Kettunen, N.; Gritsevich, M.; Hakala, T.; Särkkä, L.; Tahvonen, R. Growth and development of Norway spruce and Scots pine seedlings under different light spectra. Environ. Exp. Bot. 2016, 121, 112–120. [Google Scholar] [CrossRef]
- Bantis, F.; Radoglou, K. Morphology, development, and transplant potential of Prunus avium and Cornus sanguinea seedlings growing under different LED lights. Turk. J. Biol. 2017, 41, 314–321. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.-H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef] [Green Version]
- Craver, J.K.; Lopez, R.G. Control of morphology by manipulating light quality and daily light integral using LEDs. In LED Lighting for Urban Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 203–217. [Google Scholar]
- Pattison, P.M.; Tsao, J.Y.; Brainard, G.C.; Bugbee, B. LEDs for photons, physiology and food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Dzakovich, M.P.; Gomez, C.; Lopez, R.; Burr, J.F.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S.; et al. Light-emitting diodes in horticulture. Hortic. Rev. 2015, 43, 1–88. [Google Scholar]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Riikonen, J.; Luoranen, J. Use of short-day treatment in the production of Norway spruce mini-plug seedlings under plant factory conditions. Scand. J. For. Res. 2018, 33, 625–632. [Google Scholar] [CrossRef]
- Wallin, E.; Gräns, D.; Stattin, E.; Verhoef, N.; Mikusiński, G.; Lindström, A. Evaluating methods for storability assessment and determination of vitality status of container grown Norway spruce transplants after frozen storage. Scand. J. For. Res. 2019, 34, 417–426. [Google Scholar] [CrossRef]
- Hernandez Velasco, M. Treatments for induction of cold hardiness in Picea abies (L.) Karst. and Pinus sylvestris L. seedlings pre-cultivated under light-emitting diodes—Impact of photoperiod and temperature including energy consumption and seedling quality after cold storage. Scand. J. For. Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Lindström, A.; Hellqvist, C.; Stattin, E. Mini seedlings: A new forest regeneration system. In Proceedings of the Thin Green Line: A Symposium on the State-of-the-Art in Reforestation, Thunder Bay, ON, Canada, 26–28 July 2005; Colombo, S.J., Ed.; Ontario Forest Research Institute: Thunder Bay, ON, Canada, 2005; pp. 59–61. [Google Scholar]
- Landis, T.D. Miniplug transplants: Producing large plants quickly. In National Proceedings: Forest and Conservation Nursery Associations-2006; Proceedings RMRS-P-50; Riley, L.E., Dumroese, R.K., Landis, T.D., Eds.; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2007; Volume 50, pp. 46–53. [Google Scholar]
- Landis, T.D.; Dumroese, R.K.; Haase, D.L. Chapter 6: Outplanting. In Seedling Processing, Storage, and Outplanting—The Container Tree Nursery Manual; USDA Forest Service: Washington, DC, USA, 2010; Volume 7, pp. 154–194. [Google Scholar]
- Helenius, P.; Luoranen, J.; Rikala, R. Effect of Thawing Duration and Temperature on Field Performance of Frozen-Stored Norway Spruce Container Seedlings; Finnish Society of Forest Science: Helsinki, Finland, 2004; p. 38. [Google Scholar]
- Luoranen, J.; Pikkarainen, L.; Poteri, M.; Peltola, H.; Riikonen, J. Duration limits on field storage in closed cardboard boxes before planting of Norway spruce and Scots pine container seedlings in different planting seasons. Forests 2019, 10, 1126. [Google Scholar] [CrossRef] [Green Version]
- Riikonen, J. Pre-cultivation of Scots pine and Norway spruce transplant seedlings under four different light spectra did not affect their field performance. New For. 2016, 47, 607–619. [Google Scholar] [CrossRef]
- Smirnakou, S.; Ouzounis, T.; Radoglou, K. Effects of continuous spectrum LEDs used in indoor cultivation of two coniferous species Pinus sylvestris L. and Abies borisii-regis Mattf. Scand. J. For. Res. 2017, 32, 115–122. [Google Scholar] [CrossRef]
- Hernandez Velasco, M.; Mattsson, A. Light quality and intensity of light-emitting diodes during pre-cultivation of Picea abies (L.) Karst. and Pinus sylvestris L. seedlings—Impact on growth performance, seedling quality and energy consumption. Scand. J. For. Res. 2019, 34, 159–177. [Google Scholar] [CrossRef] [Green Version]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Bugbee, B. Effects of Radiation Quality, Intensity, and Duration on Photosynthesis and Growth; Plants, Soils, and Biometeorology Department Utah State University: Logan, UT, USA, 1994; p. 12. [Google Scholar]
- Robakowski, P. Species-specific acclimation to strong shade modifies susceptibility of conifers to photoinhibition. Acta Physiol. Plant 2005, 27, 255–263. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L.; Brown, P.H. The physiological basis of containerised tree seedling ‘transplant shock’: A review. Aust. For. 2005, 68, 112–120. [Google Scholar] [CrossRef]
- Helenius, P.; Luoranen, J.; Rikala, R.; Leinonen, K. Effect of drought on growth and mortality of actively growing Norway spruce container seedlings planted in summer. Scand. J. For. Res. 2002, 17, 218–224. [Google Scholar] [CrossRef]
- Morales, F.; Abadía, A.; AbadÞa, J. Photoinhibition and photoprotection under nutrient deficiencies, drought and salinity. In Photoprotection, Photoinhibition, Gene Regulation, and Environment; Demmig-Adams, B., Iii, W.W.A., Mattoo, A.K., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2006; pp. 65–85. ISBN 978-1-4020-3564-7. [Google Scholar]
- Long, S.P.; Humphries, S.; Falkowski, P.G. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Gilmore, A.M.; Govindjee. How higher plants respond to excess light: Energy dissipation in Photosystem II. In Concepts in Photobiology: Photosynthesis and Photomorphogenesis; Singhal, G.S., Renger, G., Sopory, S.K., Irrgang, K.-D., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 513–548. ISBN 978-94-011-4832-0. [Google Scholar]
- Krause, G.H.; Virgo, A.; Winter, K. High susceptibility to photoinhibition of young leaves of tropical forest trees. Planta 1995, 197, 583–591. [Google Scholar] [CrossRef]
- Bertamini, M.; Nedunchezhian, N. Photoinhibition of photosynthesis in mature and young leaves of grapevine (Vitis vinifera L.). Plant Sci. 2003, 164, 635–644. [Google Scholar] [CrossRef]
- Brooks, J.R.; Hinckley, T.M.; Sprugel, D.G. Acclimation responses of mature Abies amabilis sun foliage to shading. Oecologia 1994, 100, 316–324. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.S. Photosynthesis—Physiological and environmental factors. In Photobiology of Higher Plants; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 113–147. ISBN 978-0-470-85523-2. [Google Scholar]
- Carter, G.A.; Smith, W.K. Influence of shoot structure on light interception and photosynthesis in conifers. Plant Physiol. 1985, 79, 1038–1043. [Google Scholar] [CrossRef] [Green Version]
- Sprugel, D.G.; Brooks, J.R.; Hinckley, T.M. Effects of light on shoot geometry and needle morphology in Abies amabilis. Tree Physiol. 1996, 16, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, P.; Palmroth, S.; Bond, B.J.; Sprugel, D.G.; Smolander, H. Shoot structure and photosynthetic efficiency along the light gradient in a Scots pine canopy. Tree Physiol. 2001, 21, 805–814. [Google Scholar] [CrossRef] [Green Version]
- Pallardy, S.G. Physiology of Woody Plants; Academic Press: Cambridge, MA, USA, 2010; ISBN 0-08-056871-8. [Google Scholar]
- Edmond, J.B.; Edmond, J.B.; Senn, T.L.; Andrews, F.S. Fundamental of Horticulture, 4th ed.; McGraw-Hill Book Company: New York, NY, USA, 1978. [Google Scholar]
- Stenberg, P.; Kangas, T.; Smolander, H.; Linder, S. Shoot structure, canopy openness, and light interception in Norway spruce. Plant Cell Environ. 1999, 22, 1133–1142. [Google Scholar] [CrossRef]
- Leverenz, J.W.; Hinckley, T.M. Shoot structure, leaf area index and productivity of evergreen conifer stands. Tree Physiol. 1990, 6, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Watling, J.; Bittisnich, D.; Fukai, S.; Beadle, C.; Clearwater, M.; Kriedemann, P. Sunlight and plant production. In Plants in Action: Adaptation in Nature, Performance in Cultivation; Greer, D.H., Ed.; Macmillan Education AU: New York, NY, USA, 2018; ISBN 978-0-7329-4439-1. [Google Scholar]
- McDonald, M.S. Light and pigments. In Photobiology of Higher Plants; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 1–31. ISBN 978-0-470-85523-2. [Google Scholar]
- Aphalo, P.J.; Albert, A.; Björn, L.O.; McLeod, A.R.; Robson, T.M.; Rosenqvist, E. Beyond the Visible: A Handbook of Best Practice in Plant UV Photobiology; Helsingin Yliopisto: Helsinki, Finland, 2012. [Google Scholar]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Tevini, M. UV-effects on plants. In Concepts in Photobiology: Photosynthesis and Photomorphogenesis; Singhal, G.S., Renger, G., Sopory, S.K., Irrgang, K.-D., Govindjee, Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1999; pp. 588–613. ISBN 978-94-011-4832-0. [Google Scholar]
- Turunen, M.; Heller, W.; Stich, S.; Sandermann, H.; Sutinen, M.-L.; Norokorpi, Y. The effects of UV exclusion on the soluble phenolics of young Scots pine seedlings in the subarctic. Environ. Pollut. 1999, 106, 219–228. [Google Scholar] [CrossRef]
- Martz, F.; Sutinen, M.-L.; Derome, K.; Wingsle, G.; Julkunen-Tiitto, R.; Turunen, M. Effects of ultraviolet (UV) exclusion on the seasonal concentration of photosynthetic and UV-screening pigments in Scots pine needles. Glob. Chang. Biol. 2007, 13, 252–265. [Google Scholar] [CrossRef]
- Laakso, K.; Huttunen, S. Effects of the ultraviolet-B radiation (UV-B) on conifers: A review. Environ. Pollut. 1998, 99, 319–328. [Google Scholar] [CrossRef]
- Aikala, L.; Kivimaki, I. Method and Means for Acclimatizing Seedlings for Outdoor Life. U.S. Patent 13/520,805, 13 August 2015. [Google Scholar]
- Kotilainen, T.; Tegelberg, R.; Julkunen-Tiitto, R.; Lindfors, A.; Aphalo, P.J. Metabolite specific effects of solar UV-A and UV-B on alder and birch leaf phenolics. Glob. Chang. Biol. 2008, 14, 1294–1304. [Google Scholar] [CrossRef]
- Ohlsson, A.B.; Segerfeldt, P.; Lindström, A.; Borg-Karlson, A.-K.; Berglund, T. UV-B exposure of indoor-grown Picea abies seedlings causes an epigenetic effect and selective emission of terpenes. Z. Nat. C 2014, 68, 139–147. [Google Scholar]
- Turunen, M.T.; Vogelmann, T.C.; Smith, W.K. UV screening in lodgepole pine (Pinus contorta ssp. latifolia) cotyledons and needles. Int. J. Plant Sci. 1999, 160, 315–320. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L.; Holz, G.K.; Brown, P.H. Effect of shadecloth tree shelters on cold-induced photoinhibition, foliar anthocyanin and growth of Eucalyptus globulus and E. nitens seedlings during establishment. Aust. J. Bot. 2002, 50, 15–20. [Google Scholar] [CrossRef]
- Kotilainen, T.; Robson, T.M.; Hernández, R. Light quality characterization under climate screens and shade nets for controlled-environment agriculture. PLoS ONE 2018, 13, e0199628. [Google Scholar] [CrossRef] [PubMed]
- Faust, J.E.; Logan, J. Daily light integral: A research review and high-resolution maps of the United States. HortScience 2018, 53, 1250–1257. [Google Scholar] [CrossRef] [Green Version]
- Faust, J.E. Light. In Ball RedBook: Crop production; Hamrick, D., Ed.; Ball Publishing: Batavia, Indonesia, 2003; Volume 2, pp. 71–84. ISBN 978-1-883052-35-5. [Google Scholar]
- Torres, A.P.; Lopez, R.G. Measuring Daily Light Integral in a Greenhouse; Purdue University Extension; Purdue Department of Horticulture and Landscape Architecture: West Lafayette, IN, USA, 2010; p. 7. [Google Scholar]
- Hoque, E.; Remus, G. Natural UV-screening mechanisms of Norway spruce (Picea abies [L.] Karst.) needles. Photochem. Photobiol. 1999, 69, 177–192. [Google Scholar] [PubMed]
- ISO. ISO 21348:2007—Space Environment (Natural and Artificial)—Process for Determining Solar Irradiances; International Organization for Standardization: Geneva, Switzerland, 2007; p. 12. [Google Scholar]
- Sellaro, R.; Crepy, M.; Trupkin, S.A.; Karayekov, E.; Buchovsky, A.S.; Rossi, C.; Casal, J.J. Cryptochrome as a sensor of the blue/green ratio of natural radiation in Arabidopsis. Plant Physiol. 2010, 154, 401–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Both, A.-J.; Bugbee, B.; Kubota, C.; Lopez, R.G.; Mitchell, C.; Runkle, E.S.; Wallace, C. Proposed product label for electric lamps used in the plant sciences. HortTechnology 2017, 27, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Sager, J.C.; McFarlane, J.C. Radiation. In Plant Growth Chamber Handbook; Langhans, R.W., Tibbitts, T.W., Eds.; Iowa Agriculture and Home Economics Experiment Station Special Report No. 99 (SR-99) and North Central Regional Research Publication No. 340; NC-101 Regional Committee on Controlled Environment Technology and Use: Raleigh, NC, USA, 1997; Volume 99, pp. 1–29. [Google Scholar]
- Augusti 2014—Extremt Regnig I Västra GÖTALAND|SMHI. Available online: https://www.smhi.se/klimat/klimatet-da-och-nu/manadens-vader-och-vatten-sverige/manadens-vader-i-sverige/augusti-2014-extremt-regnig-i-vastra-gotaland-1.76746 (accessed on 16 January 2020).
- Swedish Meteorological and Hydrological Institute SMHI Öppna Data|Meteorologiska Observationer. Available online: http://opendata-download-metobs.smhi.se/explore/ (accessed on 4 September 2017).
- Landelius, T.; Josefsson, W.; Persson, T. STRÅNG—A System for Modelling Solar Radiation Parameters with Mesoscale Spatial Resolution; RMK; Swedish Meteorological and Hydrological Institute SMHI: Nordköping, Sweden, 2001; p. 52.
- Mattsson, A. Predicting field performance using seedling quality assessment. New For. 1997, 13, 227–252. [Google Scholar] [CrossRef]
- Haase, D.L. Understanding forest seedling quality: Measurements and interpretation. Tree Plant. Notes 2008, 52, 24–30. [Google Scholar]
- Mohammed, G.H.; Binder, W.D.; Gillies, S.L. Chlorophyll fluorescence: A review of its practical forestry applications and instrumentation. Scand. J. For. Res. 1995, 10, 383–410. [Google Scholar] [CrossRef]
- Huner, N.P.A.; Ivanov, A.G.; Sane, P.V.; Pocock, T.; Król, M.; Balseris, A.; Rosso, D.; Savitch, L.V.; Hurry, V.M.; Öquist, G. Photoprotection of Photosystem II: Reaction center quenching versus antenna quenching. In Photoprotection, Photoinhibition, Gene Regulation, and Environment; Demmig-Adams, B., Iii, W.W.A., Mattoo, A.K., Eds.; Advances in Photosynthesis and Respiration; Springer Netherlands: Dordrecht, The Netherlands, 2006; pp. 155–173. ISBN 978-1-4020-3564-7. [Google Scholar]
- L’Hirondelle, S.J.; Simpson, D.G.; Binder, W.D. Chlorophyll fluorescence, root growth potential, and stomatal conductance as estimates of field performance potential in conifer seedlings. New For. 2007, 34, 235–251. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [Green Version]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Dang, Q.L. Improving the quality and reliability of gas exchange measurements. J. Plant Physiol. Pathol. 2013, 2, 2. [Google Scholar]
- Mitchell, K.A.; Bolstad, P.V.; Vose, J.M. Interspecific and environmentally induced variation in foliar dark respiration among eighteen southeastern deciduous tree species. Tree Physiol. 1999, 19, 861–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, M.B.; Kruger, E.L.; Reich, P.B. Growth, biomass distribution and CO2 exchange of northern hardwood seedlings in high and low light: Relationships with successional status and shade tolerance. Oecologia 1993, 94, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Tjoelker, M.G.; Walters, M.B.; Vanderklein, D.W.; Buschena, C. Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light. Funct. Ecol. 1998, 12, 327–338. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]
- Vaux, D.L.; Fidler, F.; Cumming, G. Replicates and repeats—What is the difference and is it significant? A brief discussion of statistics and experimental design. EMBO Rep. 2012, 13, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Phillips, N.D. YaRrr! The Pirate’s Guide to R. APS Observer. 2017. Available online: https://www.psychologicalscience.org/observer/yarrr-the-pirates-guide-to-r (accessed on 1 February 2020).
- Aphalo, P.J. The r4photobiology suite: Spectral irradiance. UV4Plants Bull. 2015, 2015, 21–29. [Google Scholar]
- Aphalo, P.J. Using R for Photobiological Calculations. 2015. Available online: https://www.researchgate.net/publication/281627610_Using_R_for_photobiological_calculations (accessed on 1 February 2020).
- Mangiafico, S.S. Repeated Measures ANOVA. In Summary and Analysis of Extension Program Evaluation in R; Rutgers Cooperative Extension: New Brunswick, NJ, USA, 2016; pp. 653–663. [Google Scholar]
- Schwarz, C.J. Two-factor split-plot designs. In Course Notes for Intermediate Ecological Statistics; Department of Statistics and Actuarial Science, Simon Fraser University: Burnaby, BC, Canada, 2019. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W.; Winter, K.; Meyer, A.; Schreiber, U.; Pereira, J.S.; Krüger, A.; Czygan, F.-C.; Lange, O.L. Photochemical efficiency of photosystem II, photon yield of O2 evolution, photosynthetic capacity, and carotenoid composition during the midday depression of net CO2 uptake in Arbutus unedo growing in Portugal. Planta 1989, 177, 377–387. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Tjoelker, M.G.; Vanderklein, D.; Buschena, C. Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct. Ecol. 1998, 12, 395–405. [Google Scholar] [CrossRef]
- Chapman, S.R.; Carter, L.P. Crop Production: Principles and Practices; W.H. Freeman & Co Ltd.: San Francisco, CA, USA, 1976; ISBN 978-0-7167-0581-9. [Google Scholar]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, F.S. Principles of Silviculture; McGraw-Hill Book Company: New York, NY, USA, 1950; p. 414. [Google Scholar]
- Oldeman, R.A.A. Silvatic mosaics. In Forests: Elements of Silvology; Springer: Berlin/Heidelberg, Germany, 1990; pp. 388–558. ISBN 978-3-642-75213-1. [Google Scholar]
- Egbäck, S. Growth of Genetically Improved Stands of Norway Spruce, Scots Pine and Loblolly Pine. Ph.D. Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2016. [Google Scholar]
- Nelson, J.A.; Bugbee, B. Economic analysis of greenhouse lighting: Light emitting diodes vs. high intensity discharge fixtures. PLoS ONE 2014, 9, e99010. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Sowing | Transplanting Outdoors | Attributes Assessment | Time Outdoors | |
|---|---|---|---|---|
| Sowing 1 (S1) | 2014-05-05 | 2014-06-17 | 2014-09-08 | 12 weeks |
| Sowing 2 (S2) | 2014-06-17 | 2014-07-31 | 2014-09-08 | 6 weeks |
| Sowing 3 (S3) | 2015-05-25 | 2015-07-07 | 2015-10-12 | 14 weeks |
| Outdoor Treatments | Indoor Treatments | ||||
|---|---|---|---|---|---|
| Control | High Intensity | UVA 30 min | UVA 60 min | UVA 240 min | |
| No shading cloth (Control) | S1, S2, S3 | S1, S2, S3 | S1, S2 | S1, S2 | S3 |
| Shading cloth-1 week | S1, S2, S3 | S1, S2 | S1, S2 | S1, S2 | |
| Shading cloth-3 weeks | S1, S2 | S1, S2 | S1, S2 | S1, S2 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez Velasco, M.; Mattsson, A. Light Shock Stress after Outdoor Sunlight Exposure in Seedlings of Picea abies (L.) Karst. and Pinus sylvestris L. Pre-Cultivated under LEDs—Possible Mitigation Treatments and Their Energy Consumption. Forests 2020, 11, 354. https://doi.org/10.3390/f11030354
Hernandez Velasco M, Mattsson A. Light Shock Stress after Outdoor Sunlight Exposure in Seedlings of Picea abies (L.) Karst. and Pinus sylvestris L. Pre-Cultivated under LEDs—Possible Mitigation Treatments and Their Energy Consumption. Forests. 2020; 11(3):354. https://doi.org/10.3390/f11030354
Chicago/Turabian StyleHernandez Velasco, Marco, and Anders Mattsson. 2020. "Light Shock Stress after Outdoor Sunlight Exposure in Seedlings of Picea abies (L.) Karst. and Pinus sylvestris L. Pre-Cultivated under LEDs—Possible Mitigation Treatments and Their Energy Consumption" Forests 11, no. 3: 354. https://doi.org/10.3390/f11030354
APA StyleHernandez Velasco, M., & Mattsson, A. (2020). Light Shock Stress after Outdoor Sunlight Exposure in Seedlings of Picea abies (L.) Karst. and Pinus sylvestris L. Pre-Cultivated under LEDs—Possible Mitigation Treatments and Their Energy Consumption. Forests, 11(3), 354. https://doi.org/10.3390/f11030354