Intra- and Interspecific Interactions among Pioneer Trees Affect Forest-Biomass Carbon Accumulation in a Nutrient-Deficient Reclaimed Coal Mine Spoil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plot Introduction and Vegetation Survey Method
2.3. Calculation of Trees Biomass C
2.4. Spatial Arrangement of Biomass C
2.5. The Effect of Intra- and Interspecific Interaction on Tree Biomass C Accumulation
3. Results
3.1. Tree Biomass C and its Class Structures
3.2. The Spatial Arrangement of Tree Biomass C
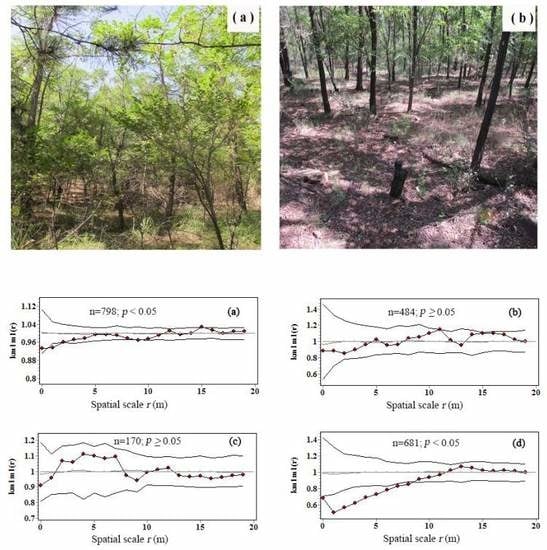
3.3. Intraspecific Interactions of Tree Biomass C
3.4. Interspecific Correlations of Tree Biomass C
4. Discussion
4.1. The Development of Forest Biomass C and RMSs
4.2. The Effect of Intra- and Interspecific Interaction on Biomass C Accumulation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ussiri, D.A.N.; Lal, R. Carbon sequestration in reclaimed minesoils. Crit. Rev. Plant Sci. 2005, 24, 151–165. [Google Scholar] [CrossRef]
- Shrestha, R.K.; Lal, R. Changes in physical and chemical properties of soil after surface mining and reclamation. Geoderma 2011, 161, 168–176. [Google Scholar] [CrossRef]
- Amichev, B.Y.; Burger, J.A.; Rodrigue, J.A. Carbon sequestration by forests and soils on mined land in the Midwestern and Appalachian coalfields of the U.S. Forest Ecol. Manag. 2008, 256, 1949–1959. [Google Scholar] [CrossRef] [Green Version]
- Adeli, A.; Brooks, J.P.; Read, J.J.; McGrew, R.; Jenkins, J.N. Post-reclamation Age Effects on Soil Physical Properties and Microbial Activity Under Forest and Pasture Ecosystems. Commun. Soil Sci. Plant Anal. 2019, 50, 20–34. [Google Scholar] [CrossRef]
- Ahirwal, J.; Maiti, S.K.; Reddy, M.S. Development of carbon, nitrogen and phosphate stocks of reclaimed coal mine soil within 8 years after forestation with Prosopis juliflora (Sw.) Dc. Catena 2017, 156, 42–50. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.M.; Liu, T.; Bai, Z.K.; Reading, L. Using computed tomography images to characterize the effects of soil compaction resulting from large machinery on three-dimensional pore characteristics in an opencast coal mine dump. J. Soils Sediments 2019, 19, 1467–1478. [Google Scholar] [CrossRef]
- Bai, Z.K.; Zhao, J.K.; Li, J.C.; Wang, W.Y.; Lu, C.E.; Ding, X.Q.; Chai, S.J.; Chen, J.J. Ecosystem damage in a large opencast coal mine-a case study on Pingshuo surface coal mine, China. Acta Ecol. Sin. 1999, 19, 870–875. (In Chinese) [Google Scholar]
- Akala, V.A.; Lal, R. Soil organic pools and sequestration rates in reclaimed minesoils in Ohio. J. Environ. Qual. 2001, 30, 2090–2104. [Google Scholar] [CrossRef]
- Ganjegunte, G.K.; Wick, A.F.; Stahl, P.D.; Vance, G.F. Accumulation and composition of total organic carbon in reclaimed coal mine lands. Land Degrad. Dev. 2009, 20, 156–175. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Z.Q.; Li, X.Z.; Wang, Y.Y.; Bai, Z.K. Characteristics of labile organic carbon fractions in reclaimed mine soils: Evidence from three reclaimed forests in the Pingshuo opencast coal mine, China. Sci. Total Environ. 2018, 613–614, 1196–1206. [Google Scholar] [CrossRef]
- Dutta, R.K.; Agrawal, M. Restoration of opencast coal mine spoil by planting exotic tree species: A case study in dry tropical region. Ecol. Eng. 2003, 21, 143–151. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Wang, L.H.; Bai, Z.K.; Pan, Z.G.; Wang, Y. Development of population structure and spatial distribution patterns of a restored forest during 17-year succession (1993–2010) in Pingshuo opencast mine spoil, China. Environ. Monit. Assess. 2015, 187, 431. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, Z.Q.; Bai, Z.K.; Wang, H.Q.; Wang, Y.Z.; Niu, S.Y. Reclamation patterns vary carbon sequestration by trees and soils in an opencast coal mine, China. Catena 2016, 147, 404–410. [Google Scholar] [CrossRef]
- Shrestha, R.K.; Lal, R. Carbon and nitrogen pools in reclaimed land under forest and pasture ecosystems in Ohio, USA. Geoderma 2010, 157, 196–205. [Google Scholar] [CrossRef]
- Dobrowolska, D.; Bolibok, L. Is climate the key factor limiting the natural regeneration of silver fir beyond the northeastern border of its distribution range? Forest Ecol. Manag. 2019, 439, 105–121. [Google Scholar] [CrossRef]
- St-Denis, A.; Kneeshaw, D.; Messier, C. Effect of predation, competition, and facilitation on tree survival and growth in abandoned fields: Towards Precision Restoration. Forests 2018, 9, 692. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.R.; Congdon, R.A.; Grice, A.C.; Clarke, P.J. Effect of fire regime on plant abundance in a tropical eucalypt savanna of north-eastern Australia. Austral Ecol. 2003, 28, 327–338. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Franklin, S.B.; Wang, Q.G.; Shi, Z.; Luo, Y.Q.; Lu, Z.J.; Zhang, J.X.; Qiao, X.J.; Jiang, M.X. Topographic and biotic factors determine forest biomass spatial distribution in a subtropical mountain moist forest. Forest Ecol. Manag. 2015, 357, 95–103. [Google Scholar] [CrossRef]
- De Castilho, C.V.; Magnusson, W.E.; De Araújo, R.N.O.; Luizão, F.J. Short-term temporal changes in tree live biomass in a central Amazonian forest, Brazil. Biotropica 2010, 42, 95–103. [Google Scholar] [CrossRef]
- Lobos-Catalán, P.; Jiménez-Castillo, M. Different patterns of biomass allocation of mature and sapling host tree in response to liana competition in the southern temperate rainforest. Austral Ecol. 2014, 39, 677–685. [Google Scholar] [CrossRef]
- Nilsen, E.T.; Huebner, C.D.; Carr, D.E.; Bao, Z. Interaction between Ailanthus altissima and Native Robiniapseudoacacia in Early Succession: Implications for Forest Management. Forests 2018, 9, 211. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, N.L.; van Mantgem, P.J.; Bunn, A.G.; Bruner, H.; Harmon, M.E.; O’Connell, K.B.; Urban, D.L.; Franklin, J.F. Causes and implications of the correlation between forest productivity and tree mortality rates. Ecol. Monogr. 2011, 81, 527–555. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.S.; Fan, X.H.; Fan, J.; Zhang, C.Y.; Xia, F.C. Effect of tree biomass Competition on the biomass partitioning of Abiesnephrolepis. Sci. Silvae Sin. 2012, 48, 14–20. (In Chinese) [Google Scholar]
- John, R.; Dalling, J.W.; Harms, K.E.; Yavitt, J.B.; Stallard, R.F.; Mirabello, M.; Hubbell, S.P.; Valencia, R.; Navarrete, H.; Vallejo, M.; et al. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA 2007, 104, 864–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirfel, K.; Heinze, S.; Hertel, D.; Leuschner, C. Effects of bedrock type and soil chemistry on thefine roots of European beech- A study on the belowground plasticity of trees. Forest Ecol. Manag. 2019, 444, 256–268. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Z.Q.; Zhang, P.F.; Chen, L.M.; Hu, T.; Niu, S.Y.; Bai, Z.K. Soil organic carbon and nitrogen pools in reclaimed mine soils under forest and cropland ecosystems in the Loess Plateau, China. Ecol. Eng. 2017, 102, 137–144. [Google Scholar] [CrossRef]
- Condit, R. Research in large, long-term tropical forest plots. Trends Ecol. Evol. 1995, 10, 18–22. [Google Scholar] [CrossRef]
- Condit, R. Tropical Forest Census Plots: Methods And results from Barro Colorado Island, Panama and a Comparison with Other Plots; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Sist, P.; Mazzei, L.; Blanc, L.; Rutishauser, E. Large trees as key elements of carbon storage and dynamics after selective logging in the Eastern Amazon. Forest Ecol. Manag. 2014, 318, 103–109. [Google Scholar] [CrossRef]
- Slik, J.W.F.; Paoli, G.; McGuire, K.; Amaral, I.; Barroso, J.; Bastian, M.; Blanc, L.; Bongers, F.; Boundja, P.; Clark, C.; et al. Large trees drive forest aboveground biomass variation in moist lowland forests across the tropics. Glob. Ecol. Biogeogr. 2013, 22, 1261–1271. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Bai, Z.K.; Zhang, Z.; Guo, D.G.; Li, J.C.; Xu, Z.G.; Pan, Z.G. Population structure and spatial distributions patterns of 17 years old plantation in a reclaimed spoil of Pingshuo opencast mine, China. Ecol. Eng. 2012, 44, 147–151. [Google Scholar] [CrossRef]
- Ma, J.; Bu, R.C.; Liu, M.; Chang, Y.; Qin, Q.; Hu, Y.M. Ecosystem carbon storage distribution between plant and soil in different forest types in Northeastern China. Ecol. Eng. 2015, 81, 353–362. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xu, J.J.; Li, M.H. Growth process of soil and water conservation forest and dynamic change of its carbon intensity. Sci. Soil Water Conserv. 2012, 10, 70–76. (In Chinese) [Google Scholar]
- Wang, N.; Wang, B.T.; Wang, R.J.; Cao, X.Y.; Wang, W.J.; Chi, L. Biomass allocation patterns and allometric models of PopulusDavidiana and Pinus Tabulaeformis Carr. in west of Shanxi Province. Bull. Soil Water Conserv. 2013, 33, 151–159. (In Chinese) [Google Scholar]
- Zhao, W.; Hu, Z.M.; Yang, H.; Zhang, L.M.; Guo, Q.; Wu, Z.Y.; Liu, D.Y.; Li, S.G. Carbon density characteristics of sparse Ulmus pumila forest and Populus simonii plantation in OnqinDaga Sandy Land and their relationships with stand age. Chin. J. Plant Ecol. 2016, 40, 318–326. (In Chinese) [Google Scholar]
- Du, H.Q.; Zhou, G.M.; Fan, W.Y.; Ge, H.L.; Xu, X.J.; Shi, Y.J.; Fan, W.L. Spatial heterogeneity and carbon contribution of aboveground biomass of moso bamboo by using geostatistical theory. Plant Ecol. 2010, 207, 131–139. [Google Scholar] [CrossRef]
- Burgos, P.; Madejon, E.; Perez-De-Mora, A.; Cabrera, F. Spatial variability of the chemical characteristics of a trace-element-contaminated soil before and after remediation. Geoderma 2006, 130, 157–175. [Google Scholar] [CrossRef]
- David, M.G.; Chao, S.; Zhang, B.; Owen, T.C. Geostatistical analyses and hazard assessment on soil lead in Silvermines area, Ireland. Environ. Pollut. 2004, 127, 239–248. [Google Scholar]
- Wälder, K.; Wälder, O. Analysing interaction effects in forests using the mark correlation function. iForest 2008, 1, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, T.; Moloney, K.A. Handbook of Spatial Point-Pattern Analysis in Ecology; CRC Press: London, UK, 2014. [Google Scholar]
- Diggle, P.J. Statistical Analysis of Spatial Point Patterns; Hodder Arnold: London, UK, 2003. [Google Scholar]
- Li, H.K.; Lei, Y.C.; Zeng, W.S. Forest carbon storage in China estimated using total volume data. Sci. Silvae Sin. 2011, 47, 7–12. (In Chinese) [Google Scholar]
- Belote, R.T.; Prisley, S.; Jones, R.H.; Fitzpatrick, M.; de Beurs, K. Forest productivity and tree diversity relationships depend on ecological context within mid-Atlantic and Appalachian forests (USA). Forest Ecol. Manag. 2011, 261, 1315–1324. [Google Scholar] [CrossRef]
- Bieng, M.A.N.; Perot, T.; de Coligny, F.; Goreaud, F. Spatial pattern of trees influences species productivity in a mature oak-pine mixed forest. Eur. J. Forest Res. 2013, 132, 841–850. [Google Scholar] [CrossRef] [Green Version]
- Ratcliffe, S.; Holzwarth, F.; Nadrowski, K.; Levick, S.; Wirth, C. Tree neighbourhood matters-Tree species composition drives diversity-productivity patterns in a near-natural beech forest. Forest Ecol. Manag. 2015, 335, 225–234. [Google Scholar] [CrossRef]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Gunatilleke, N.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; LaFrankie, J.V.; et al. Spatial patterns in the distribution of tropical tree species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Fraver, S.; D’Amato, A.W.; Bradford, J.B.; Jonsson, B.G.; Jonsson, M.; Esseen, P.A. Tree growth and competition in an old-growth Piceaabies forest of boreal Sweden: Influence of tree spatial patterning. J. Veg. Sci. 2014, 25, 374–385. [Google Scholar] [CrossRef]
- Law, R.; Illian, J.; Burslem, D.F.R.P.; Gratzer, G.; Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N. Ecological information from spatial patterns of plants: Insights from point process theory. J. Ecol. 2009, 97, 616–628. [Google Scholar] [CrossRef]
- Hao, M.H.; Zhang, Z.H.; Zhao, S.S.; Zhang, C.Y.; Zhao, X.M. Spatial autocorrelation patterns of tree growth in a coniferous and broad-leaved mixed forest in Jiaohe of Jilin province. Acta Ecol. Sin. 2017, 37, 1922–1930. (In Chinese) [Google Scholar]
- Yan, Y.; Xia, M.; Fan, S.; Zhan, M.; Guan, F. Detecting the Competition between Moso Bamboos and Broad-Leaved Trees in Mixed Forests Using a Terrestrial Laser Scanner. Forests 2018, 9, 520. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.Q.; Wu, Z.F.; Xu, Z.Y.; Yin, Z.X.; Wang, Q.Z.; Wang, X.W.; Wang, J.P.; Wang, L.Y.; Guo, D.G. Study on Species Composition and spatial pattern changes of Robiniapseudoacacia + Pinus tabuliformis mixed forest plot in opencast coal mine reclamation area. Environ. Sustain. Dev. 2015, 6, 31–35. (In Chinese) [Google Scholar]
- Gradel, A.; Ammer, C.; Ganbaatar, B.; Nadaldorj, O.; Dovdondemberel, B.; Wagner, S. On the Effect of Thinning on Tree Growth and Stand Structure of White Birch (Betula platyphyllaSukaczev) and Siberian Larch (LarixsibiricaLedeb.) in Mongolia. Forests 2017, 8, 105. [Google Scholar] [CrossRef]






| Biomass (kg) | Tree Species | ||
|---|---|---|---|
| R. pseudoacacia | P. tabuliformis | U. pumila | |
| Total | |||
| Stem | – | ||
| Branch | – | ||
| Leaf | – | ||
| Root | – | ||
| Item | Tree Species | |||
|---|---|---|---|---|
| Planted R. pseudoacacia | Seeded-in R. pseudoacacia | P. tabuliformis | U. pumila | |
| Tree number (stem) | 798 | 484 | 170 | 681 |
| Diameter (cm) | ||||
| Min | 7.00 | 0.31 | 1.24 | 0.30 |
| Mean | 10.41 | 2.99 | 7.00 | 1.74 |
| Max | 19.98 | 6.99 | 12.75 | 6.86 |
| C (kg per stem) | ||||
| Min | 8.46 | 0.01 | 1.62 | 0.02 |
| Mean | 23.25 | 1.94 | 9.03 | 1.83 |
| Max | 102.53 | 8.43 | 20.95 | 51.10 |
| kurtosis | 3.40 | 0.27 | −0.446 | 28.83 |
| Skewness | 1.43 | 1.32 | 0.402 | 5.03 |
| P | 0.00 | 0.00 | 0.27 | 0.00 |
| Species | Best Model | Nugget | Sill | Range (m) | Nugget/Sill (%) | Residual Sum of Squares |
|---|---|---|---|---|---|---|
| Planted R. pseudoacacia | Linear | 0.217 | 2.717 | 60.15 | 7.9 | 0.21 |
| Seeded-in R. pseudoacacia | Exponential | 0.270 | 2.441 | 4.20 | 11.06 | 0.13 |
| P. tabuliformis | Exponential | 1.640 | 12.24 | 3.60 | 13.39 | 0.15 |
| U. pumila | Exponential | 0.235 | 0.422 | 22.5 | 55.7 | 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Ren, Y.; Gao, G.; Zhao, Z.; Niu, S. Intra- and Interspecific Interactions among Pioneer Trees Affect Forest-Biomass Carbon Accumulation in a Nutrient-Deficient Reclaimed Coal Mine Spoil. Forests 2020, 11, 819. https://doi.org/10.3390/f11080819
Yuan Y, Ren Y, Gao G, Zhao Z, Niu S. Intra- and Interspecific Interactions among Pioneer Trees Affect Forest-Biomass Carbon Accumulation in a Nutrient-Deficient Reclaimed Coal Mine Spoil. Forests. 2020; 11(8):819. https://doi.org/10.3390/f11080819
Chicago/Turabian StyleYuan, Ye, Yingxiang Ren, Guoqing Gao, Zhongqiu Zhao, and Shuye Niu. 2020. "Intra- and Interspecific Interactions among Pioneer Trees Affect Forest-Biomass Carbon Accumulation in a Nutrient-Deficient Reclaimed Coal Mine Spoil" Forests 11, no. 8: 819. https://doi.org/10.3390/f11080819
APA StyleYuan, Y., Ren, Y., Gao, G., Zhao, Z., & Niu, S. (2020). Intra- and Interspecific Interactions among Pioneer Trees Affect Forest-Biomass Carbon Accumulation in a Nutrient-Deficient Reclaimed Coal Mine Spoil. Forests, 11(8), 819. https://doi.org/10.3390/f11080819