Oak Decline Caused by Biotic and Abiotic Factors in Central Europe: A Case Study from the Czech Republic
Abstract
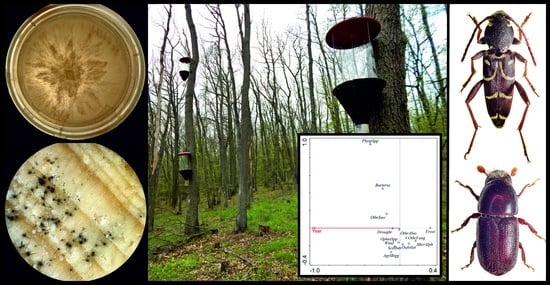
:1. Introduction
2. Materials and Methods
2.1. Review Methodology and Data Analyses
2.2. Study Plots
2.3. Insect Trapping
2.4. Sampling, Isolation, and Identification of Ophiostoma and Phytophthora
2.5. A New Modified Model of Factors Involved in Oak Decline
3. Results
3.1. Insect Pests
3.2. Presence of Ophiostoma and Phytophthora
3.3. A New Modified Model of Abiotic and Biotic Factors Involved in Oak Decline
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Study Plot | Pístecký les | Česká Skalice | Nižbor | Český Kras | Lásenice | Tvořihráz | Total | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family | Species | WT | RC | WT | RC | WT | RC | WT | RC | WT | RC | WT | RC | WT | RC |
| Bostrichidae | Bostrichus capucinus | 1 | 1 | 0.0 | |||||||||||
| Lichenophanes varius | 0.8 | 0.2 | 0 | 1.0 | |||||||||||
| Xylopertha retusa | 1 | 1 | 0.0 | ||||||||||||
| Buprestidae | Agrilus angustulus | 1 | 0.3 | 1 | 0.6 | 1 | 3 | 0.9 | |||||||
| Agrilus biguttatus | 3 | 7 | 5.3 | 45 | 1 | 0.6 | 56 | 5.8 | |||||||
| Agrilus graminis | 0.5 | 0.3 | 0.9 | 0 | 1.7 | ||||||||||
| Agrilus hastulifer | 9 | 9 | 0.0 | ||||||||||||
| Agrilus laticornis | 0.0 | 1 | 1 | 0.0 | |||||||||||
| Agrilus obscuricollis | 1 | 1 | 0.0 | ||||||||||||
| Agrilus sulcicollis | 2 | 0.4 | 7 | 5.1 | 42 | 0.4 | 1 | 52 | 5.9 | ||||||
| Coraebus undatus | 1 | 1 | 0.0 | ||||||||||||
| Cerambycidae | Alosterna tabacicolor | 0.6 | 0 | 0.6 | |||||||||||
| Anaglyptus mysticus | 4 | 1 | 2.3 | 5 | 2.3 | ||||||||||
| Anoplodera rufipes | 4 | 0.6 | 4 | 0.6 | |||||||||||
| Cerambyx scopolii | 1 | 1 | 0.0 | ||||||||||||
| Clytus arietis | 1 | 1 | 2 | 0.0 | |||||||||||
| Clytus tropicus | 9 | 1 | 10 | 0.0 | |||||||||||
| Cortodera humeralis | 1 | 10 | 13 | 18 | 42 | 0.0 | |||||||||
| Dinoptera collaris | 0.3 | 2 | 21.4 | 2 | 21.7 | ||||||||||
| Exocentrus adspersus | 0.1 | 0.2 | 2 | 2 | 0.3 | ||||||||||
| Grammoptera abdominalis | 1 | 1 | 1 | 3 | 0.0 | ||||||||||
| Grammoptera ruficornis | 1 | 1 | 0.0 | ||||||||||||
| Grammoptera ustulata | 1 | 1 | 0.0 | ||||||||||||
| Chlorophorus figuratus | 1 | 1 | 0.0 | ||||||||||||
| Leiopus linnei | 2 | 3 | 1.9 | 5 | 0.4 | 1 | 11 | 2.3 | |||||||
| Leiopus nebulosus | 0.4 | 1 | 0.2 | 0.7 | 1 | 1.2 | |||||||||
| Mesosa nebulosa | 1 | 1 | 0.0 | 1 | 3 | 0.0 | |||||||||
| Necydalis major | 1 | 1 | 0.0 | ||||||||||||
| Pachytodes cerambyciformis | 1 | 1 | 0.0 | ||||||||||||
| Pachytodes erraticus | 1 | 1 | 0.0 | ||||||||||||
| Pedostrangalia revestita | 1 | 6 | 7 | 0.0 | |||||||||||
| Phymatodes alni | 1 | 0.2 | 0.6 | 3 | 4 | 0.9 | |||||||||
| Phymatodes pusillus pusillus | 2 | 0.0 | 2 | 0.0 | |||||||||||
| Phymatodes testaceus | 11 | 0.6 | 4 | 17 | 6.9 | 2 | 13 | 47 | 7.5 | ||||||
| Plagionotus detritus | 29 | 0.6 | 7 | 36 | 0.6 | ||||||||||
| Pogonocherus hispidus | 1 | 1 | 0.0 | ||||||||||||
| Pyrrhidium sanguineum | 0.2 | 2 | 1 | 7.7 | 3 | 7.9 | |||||||||
| Rhagium sycophanta | 2 | 2 | 1 | 4 | 9 | 0.0 | |||||||||
| Stenocorus quercus | 7 | 8 | 15 | 0.0 | |||||||||||
| Trichoferus pallidus | 9 | 9 | 0.0 | ||||||||||||
| Xylotrechus antilope | 1.0 | 9 | 13.2 | 589 | 1.0 | 4.4 | 6 | 604 | 19.5 | ||||||
| Curculionidae | Dryocoetes villosus villosus | 2 | 3 | 5 | 0.0 | ||||||||||
| Gasterocercus depressirostris | 1 | 1 | 7.1 | 83 | 46.8 | 25 | 110 | 53.9 | |||||||
| Magdalis exarata | 0.0 | 0 | 0.0 | ||||||||||||
| Magdalis flavicornis | 1 | 1 | 0.0 | ||||||||||||
| Platypus cylindrus | 1 | 1 | 0.0 | ||||||||||||
| Scolytus intricatus | 4 | 2 | 3.8 | 22 | 94.8 | 140 | 114.0 | 1.4 | 18 | 186 | 214.1 | ||||
| Trypodendron domesticum | 1 | 1 | 0.0 | ||||||||||||
| Xyleborinus alni | 1 | 1 | 0.0 | ||||||||||||
| Xyleborus dispar | 1 | 1 | 0.0 | ||||||||||||
| Xyleborus dryographus | 22 | 11.3 | 1 | 23 | 11.3 | ||||||||||
| Xyleborus germanus | 1 | 1 | 0.0 | ||||||||||||
| Xyleborus monographus | 1 | 45 | 4.1 | 6 | 9.2 | 37 | 0.6 | 32.7 | 65 | 1.7 | 154 | 48.3 | |||
| Xyleborus saxeseni | 1 | 2 | 6 | 9 | 0.0 | ||||||||||
| Hymenoptera | Xiphydria longicollis | 1 | 11.0 | 7 | 0.6 | 8 | 11.6 | ||||||||
| Total WT | 18 | 72 | 89 | 1068 | 5 | 203 | 1455 | ||||||||
| Total RC | 0 | 12.7 | 148.9 | 134.0 | 119.7 | 4.5 | 419.9 | ||||||||
References
- Delatour, C. Les dépérissements de chênes en Europe. Biol. For. 1983, 35, 265–282. [Google Scholar] [CrossRef] [Green Version]
- Schütt, P. Oak decline in central and eastern Europe. A critical review of a little understood phenomenon. In Recent Advances in Studies on Oak Decline, Proceedings of the International Congress, Brindisi, Italy, 13–18 September, 1992; Luisi, N., Vannini, A., Eds.; Dipartimento di Pathologia Vegetale Università Degli Studi; International Union of Forestry Research Organizations (IUFRO): Bari, Italia, 1993; pp. 235–240. [Google Scholar]
- Ragazzi, A.; Vagniluca, S.; Moricca, S. European expansion of oak decline, involved microorganisms and methodological approaches. Phytopathol. Mediterr. 1995, 34, 207–226. [Google Scholar]
- Brasier, C.M. Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Ann. Sci. For. 1996, 53, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Oszako, T. Oak declines in Europe’s forests—History, causes and hypothesis. In Recent Advances on Oak Health in Europe, Proceedings of the International Scientific Conference, Warsaw, Poland, 22–24 November 1999; Oszako, T., Delatour, C., Eds.; Forest Research Institute: Warsaw, Poland, 2000; pp. 11–40. [Google Scholar]
- Thomas, F.M. Recent advances in cause-effect research on oak decline in Europe. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2008, 3, 1–12. [Google Scholar] [CrossRef]
- Millers, I.; Shriner, D.S.; Rizzo, D. History of Hardwood Decline in the Eastern United States; General Technical Report, NE-126; U.S. Department of Agriculture, Forest Service: Broomall, PA, USA, 1989; pp. 1–78. [CrossRef]
- Attarod, P.; Rostami, F.; Dolatshahi, A.; Sadeghi, S.M.M.; Zahedi Amiri, G.; Bayramzadeh, V. Do Changes in Meteorological Parameters and Evapotranspiration Affect Declining Oak Forests of Iran? J. For. Sci. 2016, 62, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Oleksyn, J.; Przybil, K. Oak decline in the Soviet Union—Scale and hypotheses. Eur. J. Plant Pathol. 1987, 17, 321–336. [Google Scholar] [CrossRef]
- Siwecki, R.; Liese, W. Oak Decline in Europe. In Proceedings of the International Symposium, Kórnik, Poland, 15–18 May 1990; Polish Academy of Sciences Institute of Dendrology: Kórnik, Poland, 1991; pp. 1–360. [Google Scholar]
- Luisi, N.; Lerario, P.; Vannini, A. Recent Advances in Studies on Oak Decline. In Proceedings of the International Congress, Brindisi, Italy, 13–18 September 1992; Dipartimento di Pathologia Vegetale Università Degli Studi; International Union of Forestry Research Organizations (IUFRO): Bari, Italia, 1993; pp. 1–541. [Google Scholar]
- Führer, E. Oak decline in Central Europe: A synopsis of hypotheses. In Proceedings of the Population Dynamics, Impacts, and Integrated Management of Forest Defoliating Insects, Banská Štiavnica, Slovakia, 18–23 August 1996; McManus, M.L., Liebhold, A.M., Eds.; USDA Forest Service General Technical Report NE-247. USDA: Washington, DC, USA, 1998; pp. 7–24. [Google Scholar]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Manion, P.D. Tree Disease Concepts, 2nd ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1981. [Google Scholar]
- Čapek, M.; Findo, S.; Brutovský, D. Možnosti biologickej ochrany proti vektorom hromadného hynutia dubov. In Problematika Hynutia Dubov na Slovensku, Vedecké práce VÚLH vo Zvolene; Príroda v Bratislave: Bratislava, Slovakia, 1987; Volume 36, pp. 169–182. [Google Scholar]
- Nienhaus, F. Viren und primitive Prokaryonten in Eichen. Osterr. Forstztg. 1987, 3, 64–65. [Google Scholar]
- Ahrens, U.; Seemüller, E. Detection of mycoplasmalike organisms in declining oaks by polymerase chain reaction. Eur. J. Plant Pathol. 1994, 24, 55–63. [Google Scholar] [CrossRef]
- Šafar, J. Problem fizioloških, ekoloških i ekonomskih karakteristika kasnoga i ranog hrasta lužnjaka. Sumar. List 1966, 90, 503–515. [Google Scholar]
- Dantec, C.F.; Ducasse, H.; Capdevielle, X.; Fabreguettes, O.; Delzon, S.; Desprez-Loustau, M.-L. Escape of spring frost and disease through phenological variations in oak populations along elevation gradients. J. Ecol. 2015, 103, 1044–1056. [Google Scholar] [CrossRef] [Green Version]
- Puchałka, R.; Koprowski, M.; Gričar, J.; Przybylak, R. Does tree-ring formation follow leaf phenology in Pedunculate oak (Quercus robur L.)? Eur. J. For. Res. 2017, 136, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Utkina, I.A.; Rubtsov, V.V. Studies of Phenological Forms of Pedunculate Oak. Contemp. Probl. Ecol. 2017, 10, 804–811. [Google Scholar] [CrossRef]
- Wesołowski, T.; Rowiński, P. Late leaf development in pedunculate oak (Quercus robur): An antiherbivore defence? Scand. J. For. Res. 2008, 23, 386–394. [Google Scholar] [CrossRef]
- Georgevitch, P. Armillaria mellea (Vahl) Quel. cause du dessechement forets de chene en Yougoslavie. Compte-Rendus Achademic Sci. 1926, 182, 289–491. [Google Scholar]
- Georgevitch, P. Ceratostomella quercus n. sp., Ein parasite der slawonischen Eichen. Biol. Gen. 1927, 3, 245–252. [Google Scholar]
- Marcu, G. Ursachen des Eichensterbens in Rumänien und Gegenmassnahmen. Österr. Forstz 1987, 98, 53–54. [Google Scholar]
- Brasier, C.M. Phytophthora cinnamomi as a contributory factor on European oak declines. In Recent Advances in Studies on Oak Decline, Proceedings of the International Congress, Brindisi, Italy, 13–18 September 1992; Luisi, N., Vannini, A., Eds.; Dipartimento di Patholog ia Vegetale Università Degli Studi; International Union of Forestry Research Organizations (IUFRO): Bari, Italia, 1993; pp. 49–58. [Google Scholar]
- Andersson, M.; Milberg, P.; Bergman, K.O. Low pre-death growth rates of oak (Quercus robur L.)—Is oak death a long-term process induced by dry years? Ann. For. Sci. 2011, 68, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Balci, Y.; Halmschlager, E. Incidence of Phytophthora species in oak forests in Austria and their possible involvement in oak decline. For. Pathol 2003, 33, 157–174. [Google Scholar] [CrossRef]
- Hansen, E.; Delatour, C. Phytophthora species in oak forests of north-east France. Ann. For. Sci. 1999, 56, 539–547. [Google Scholar] [CrossRef]
- Jung, T.; Blaschke, H.; Neumann, P. Isolation, identification and pathogenicity of Phytophthora species from declining oak stands. Eur. J. Plant Pathol. 1996, 26, 253–272. [Google Scholar] [CrossRef]
- Jung, T.; Blaschke, H.; Oßwald, W. Involvement of soilborne Phytophthora species in Central European oak decline and the effect of site factors on the disease. Plant Pathol. 2000, 49, 706–718. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Barzanti, G.P.; Bianco, M.C.; Ragazzi, A.; Capretti, P.; Paoletti, E.; Luisi, N.; Anselmi, N.; Vannini, A. Occurrence of Phytophthora species in oak stands in Italy and their association with declining oak trees. For. Pathol 2002, 32, 19–28. [Google Scholar] [CrossRef]
- Biosca, E.G.; Gonzalez, R.; Lopez-Lopez, M.J.; Soria, S.; Monton, C.; Perez-Laorga, E.; Lopez, M.M. Isolation and characterization of Brenneria quercina, causal agent for bark canker and drippy nut of Quercus spp. in Spain. Phytopathology 2003, 93, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, N.; Jeger, M.; Kirk, S.; Williams, D.; Xu, X.; Pautasso, M.; Denman, S. Acute oak decline and Agrilus biguttatus: The co-occurrence of stem bleeding and D-shaped emergence holes in Great Britain. Forests 2017, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gentilesca, T.; Oliva, J.; Redondo, M.A.; Ripullone, F. Drought and Phytophthora are associated with the decline of oak species in southern Italy. Front. Plant Sci. 2018, 9, 1595. [Google Scholar] [CrossRef]
- Conte, A.L.; Di Pietro, R.; Iamonico, D.; Di Marzio, P.; Cillis, G.; Lucia, D.; Fortini, P. Oak decline in the Mediterranean Basin: A Study Case from the Southern Apennines (Italy). Plant Sociol. 2019, 56, 69–80. [Google Scholar] [CrossRef]
- Correia, A.C.; Galla, A.; Nunes, A.; Pereira, J.S. Ecological interactions between cork oak (Quercus suber L.) and stone pine (Pinus pinea L.): Results from a pot experiment. Forests 2019, 9, 534. [Google Scholar] [CrossRef] [Green Version]
- Denman, S.; Brown, N.; Kirk, S.; Jeger, M.; Webber, J. A description of the symptoms of acute oak decline in Britain and a comparative review on causes of similar disorders on oak in Europe. Forestry 2014, 87, 535–551. [Google Scholar] [CrossRef] [Green Version]
- Doležal, J.; Mazůrek, P.; Klimešová, J. Oak decline in southern Moravia: The association between climate change and early and late wood formation in oaks. Preslia 2010, 82, 289–306. [Google Scholar]
- Donaubauer, E. Auftreten von Krankheiten und Schädlingen der Eiche und ihr Bezug zum Eichensterben. Osterreichische Forstztg. 1987, 3, 46. [Google Scholar]
- Frisullo, S.; Lima, G.; Magnano di San Lio, G.; Camele, I.; Melissano, L.; Puglisi, I.; Pane, A.; Agosteo, G.E.; Prudente, L.; Cacciola, S.O. Phytophthora cinnamomi involved in the decline of holm oak (Quercus ilex) stands in southern Italy. For. Sci. 2018, 64, 290–298. [Google Scholar] [CrossRef]
- Gibbs, J.N.; Greig, B.J.W. Biotic and abiotic factors affecting the dying back of pedunculate oak Quercus robur L. Forestry 1997, 70, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, G.; Blank, R. Winterfrost, Kahlfraß und Pracht Käferbefall als Faktoren im Ursachenkomplex des Eichensterbens in Norddeutschland. Forst Und Holz 1992, 47, 443–452. [Google Scholar]
- Jurc, D. Oak Decline and the Status of Ophiostoma spp. on Oak in Europe (Yugoslavia). Bull. OEPP/EPPO Bull. 1990, 20, 419–422. [Google Scholar]
- Jurc, M.; Bojović, S.; Komjanc, B.; Krč, J. Xylophagous entomofauna in branches of oak (Quercus spp.) and its significance for oak health in the Karst region of Slovenia. Biologia 2009, 64, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Klepáč, D. Les forêts de Chêne en Slavonie. Rev. For. Fr. 1981, 33, 86–106. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, T. Oak Decline and the Status of Ophiostoma spp. on Oak in Europe (Poland). Bull. OEPP/EPPO Bull. 1990, 20, 417–418. [Google Scholar]
- Losseau, J.; Jonard, M.; Vincke, C. Pedunculate oak decline in southern Belgium: A long-term process highlighting the complex interplay among drought, winter frost, biotic attacks, and masting. Can. J. For. Res. 2020, 50, 380–389. [Google Scholar] [CrossRef]
- Moraal, L.G.; Hilszczánski, J. The oak buprestid beetle, Agrilus biguttatus (F.) (Col., Buprestidae), a recent factor in oak decline in Europe. Anz. Für Schädlingskunde 2000, 73, 134–138. [Google Scholar]
- Mora-Sala, B.; Berbegal, M.; Abad-Campos, P. The use of qPCR reveals a high frequency of Phytophthora quercina in two Spanish holm oak areas. Forests 2018, 9, 697. [Google Scholar] [CrossRef] [Green Version]
- Oosterbaan, A. Oak Decline and the Status of Ophiostoma spp. on Oak in Europe (Netherlands). Bull. OEPP/EPPO Bull. 1990, 20, 414–417. [Google Scholar]
- Redondo, M.Á.; Thomsen, I.M.; Oliva, J. First report of Phytophthora uniformis and P. plurivora causing stem cankers on Alnus glutinosa in Denmark. Plant Dis. 2017, 101, 512. [Google Scholar] [CrossRef]
- Rossnev, B.; Petkov, P.; Georgiev, D. Importance and character of the tracheomycotic disease in the oak forests of Bulgaria. Nauka Za Gorata 1994, 31, 49–54. [Google Scholar]
- Ruffner, B.; Schneider, S.; Meyer, J.B.; Queloz, V.; Rigling, D. First report of acute oak decline disease of native and nonnative oaks in Switzerland. New Dis. Rep. 2020, 41, 18. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Gómez, F.J.; Pérez-de-Luque, A.; Navarro-Cerrillo, R.M. The involvement of Phytophthora root rot and drought stress in holm oak decline: From ecophysiology to microbiome influence. Curr. For. Rep. 2019, 5, 251–266. [Google Scholar] [CrossRef]
- Selochnik, N.N.; Pashenova, N.V.; Sidorov, E.; Wingfield, M.J.; Linnakoski, R. Ophiostomatoid fungi and their roles in Quercus robur die-back in Tellermann forest, Russia. Silva Fenn. 2015, 49, 16. [Google Scholar] [CrossRef] [Green Version]
- Siwecki, R.; Ufnalski, K. Review of oak stand decline with special reference to the role of drought in Poland. Eur. J. Plant Pathol. 1998, 28, 99–112. [Google Scholar] [CrossRef]
- Sohar, K.; Helama, S.; Läänelaid, A.; Raisio, J.; Tuomenvirta, H. Oak decline in a southern Finnish forest as affected by a drought sequence. Geochronometria 2014, 41, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Sonesson, K.; Drobyshev, I. Recent advances on oak decline in southern Sweden. Ecol. Bull. 2010, 53, 197–208. [Google Scholar]
- Spaič, I. O sušenju hrastika. Šumarski List. 1974, 78, 273–284. [Google Scholar]
- Stojanović, D.; Levanič, T.; Matović, B.; Bravo-Oviedo, A. Climate change impact on a mixed lowland oak stand in Serbia. Ann. Silvic. Res. 2015, 39, 94–99. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Celma, L.; Ruņģis, D.E.; Bokuma, G. First report on Brenneria goodwinii and Gibbsiella quercinecans bacteria, detected on weaken oak trees in Poland. Balt. For. 2021, 27, 1. [Google Scholar] [CrossRef]
- Tomiczek, C. Oak decline in Austria and Europe. Arboric. J. 1993, 19, 71–73. [Google Scholar] [CrossRef]
- Vannini, A.; Luisi, N. Oak Decline and the Status of Ophiostoma spp. on Oak in Europe (Italy). Bull. OEPP/EPPO Bull. 1990, 20, 413–414. [Google Scholar]
- Yde-Andersen, A. Oak Decline and the Status of Ophiostoma spp. on Oak in Europe. Bull. OEPP/EPPO Bull. 1990, 20, 405–422. [Google Scholar]
- Zumr, V. Hmyz jako potenciální vektor tracheomykózního odumírání dubů. In Aktuální Problémy Ochrany Dřevin: Sborník Referátů z Odb. Semináře; Čížková, D., Švecová, M., Eds.; Ministerstvo Životního Prostředí ČR: Prague, Czech Republic, 1995; Volume 1, pp. 89–92. [Google Scholar]
- Petrescu, M. Dieback of oak in Romania. Eur. J. For. Path. 1974, 4, 222–227. [Google Scholar] [CrossRef]
- Oszako, T. Protection of Forests Against Pest Insects and Diseases. European Oak Decline Study Case; Forests Research Institute (IBL): Warsaw, Poland, 2004; ISBN 83-87-647-37-3. [Google Scholar]
- Varga, F. Disease and dying of trees in Hungarian oak pedunculate stands. Osterr. Forstztg. 1987, 3, 57–58. [Google Scholar]
- Yakoylev, A.I.; Yakovlev, S.A. Oak decline in the Middle Povolzhje region of Russia. Recent Advances on Oak Health in Europe. In Proceedings of the International Scientific Conference, Warsaw, Poland, 22–24 November 1999; Oszako, T., Delatour, C., Eds.; Forest Research Institute: Warsaw, Poland, 2000; pp. 73–82. [Google Scholar]
- Uroševič, B. Tracheomycotic diseases in oak. Commun. Inst. For. Cechoslov. 1957, 13, 85–100. [Google Scholar]
- Igmandy, Z.; Pagony, H.; Szoutagh, P.; Varga, F. A report on the mortality in national sessile oak stands 1978–1983. Acta Fac. For. 1985; 5–16. [Google Scholar]
- Příhoda, A. Hynutí dubů ve středních Čechách. Bohemia Cent. 1990, 19, 81–90. [Google Scholar]
- Jančařík, V. Tracheomykózní onemocnění lesních dřevin—Realita a hrozba. In Proceedings of the Aktuální Problémy Ochrany Dřevin. Sborník Referátů z Odb. Semináře, Prachatice, Czech Republic, 3–4 October 1995; Čížková, D., Švecová, M., Eds.; Ministerstvo Životního Prostředí ČR: Prague, Czech Republic, 1995; Volume 2, pp. 5–17. [Google Scholar]
- Švecová, M.; Skalický, V. Problematika hub řádu Ophiostomatales a jejich výskyt v českých zemích. In Proceedings of the Ophiostomatales—Výsledky Současného Taxonomického a Fytopatologického Výzkumu. Sborník Referátů, Prague, Czech Republic, 29 May 1991; Holubová, V., Prášil, K., Eds.; Československá Vědecká Společnost pro Mykologii při ČSAV: Prague, Czech Republic, 1992; pp. 19–29. [Google Scholar]
- Kubátová, A.; Prášil, K. Ophiostomatální a další mikroskopické houby lesních dřevin s příznaky tracheomykózního onemocnění. In Proceedings of the Aktuální Problémy Ochrany Dřevin. Sborník Referátů, Prachatice, Czech Republic, 3–4 October 1995; Čížková, D., Švecová, M., Eds.; Ministerstvo Životního Prostředí ČR: Prague, Czech Republic, 1995; Volume 2, pp. 89–92. [Google Scholar]
- Kowalski, T.; Bartnik, C. Ceratocystis Species on Quercus robur with Oak Decline Symptoms in Southern Poland. Bull. OEPP/EPPO Bull. 1990, 20, 221–228. [Google Scholar] [CrossRef]
- Kowalski, T.; Butin, H. Taxonomie bekannter und neuer Ceratocystis-Arten an Eiche (Quercus robur L.). J. Phytopathol. 1989, 124, 236–248. [Google Scholar] [CrossRef]
- Kowalski, T.; Domanski, S. Preliminary results of artificial inoculations of Quercus robur L. with different species of Ceratocystis. Oak Decline in Europe. In Proceedings of the International Symposium, Kórnik, Poland, 15–18 May 1990; Siwecki, R., Liese, W., Eds.; Polish Academy of Sciences Institute of Dendrology: Kórnik, Poland, 1991; pp. 93–104. [Google Scholar]
- Rohde, T. Beitrag zur Kenntnis Einer Krebsartigen Eichenkrankheit und Ihrer Pilzflora. Ph.D. Thesis, M. &. H. Schaper, Hannover, Germany, 1936. [Google Scholar]
- Leontovyč, R.; Gontková, E. Napadnutie, náchylnosť a odolnosť jednotlivých druhov k hromadnému hynutiu dubov. In Problematika Hynutia Dubov na Slovensku, Vedecké Práce VÚLH vo Zvolene; Príroda v Bratislave: Bratislava, Slovakia, 1987; Volume 36, pp. 211–232. [Google Scholar]
- Beranová, J. Umělá infekce dubových sazenic houbovými původci tracheomykózního onemocnění. Zprávy Lesn. Výzkumu 1989, 3, 38–41. [Google Scholar]
- Oszako, T. Influence of water stress, defoliation and inoculation with Ophiostoma querci on pedunculate oak seedlings. Folia For. Pol. A For. 1997, 39, 5–15. [Google Scholar]
- Novotný, D. Ophiostomatales a lesní dřeviny (zvláště dub). In Proceedings of the Houby a les, Sborník z Konference s Mezinárodní Účastí, Brno, Czech Republic, 3–4 June 1999; MZLU: Brno, Czech Republic, 1999; pp. 89–94. [Google Scholar]
- Somogyi, Z. Oak decline in Hungary: Case study. Recent Advances on Oak Health in Europe. In Proceedings of the International Scientific Conference, Warsaw, Poland, 22–24 November 1999; Oszako, T., Delatour, C., Eds.; Forest Research Institute: Warsaw, Poland, 2000; pp. 73–82, 91–103. [Google Scholar]
- Novotný, D. Příspěvek k mykoflóře dubů s tracheomykózními příznaky. In Proceedings of the Aktuální problémy ochrany dřevin. Sborník referátů, Prachatice, Czech Republic, 3–4 October 1995; Čížková, D., Švecová, M., Eds.; Ministerstvo Životního Prostředí ČR: Prague, Czech Republic, 1995; Volume 2, pp. 52–64. [Google Scholar]
- Novotný, D. Endofyty a ophiostomatální houby ve vztahu k listnatým dřevinám. Zprávy Lesn. Výzkumu 2003, 48, 126–129. [Google Scholar]
- Przybyl, K. On the pathogenicity of Ophiostoma piceae. Oak decline in Europe. In Proceedings of the International Symposium, Kórnik, Poland, 15–18 May 1990; Siwecki, R., Liese, W., Eds.; Polish Academy of Sciences Institute of Dendrology: Kórnik, Poland, 1991; pp. 83–89. [Google Scholar]
- Malloch, D.; Blackwell, M. Dispersal biology of the ophiostomatoid fungi. In Ceratocystis and Ophiostoma: Taxonomy, Ecology, and Pathogenicity; Wingfield, M.J., Seifert, K.A., Webber, J.F., Eds.; American Phytopathological Society Press: St Paul, MN, USA, 1993; pp. 195–206. ISBN 978-0890541562. [Google Scholar]
- Wingfield, M.J.; Barnes, I.; De Beer, Z.W.; Roux, J.; Wingfield, B.D.; Taerum, S.J. Novel associations between ophiostomatoid fungi, insects and tree hosts: Current status—Future prospects. Biol. Invasions 2017, 19, 3215–3228. [Google Scholar] [CrossRef]
- Fassatiová, O. Houby v chodbách kůrovců. Česká Mykol. 1954, 8, 138–143. [Google Scholar]
- Heško, J. Diagnostické znaky tracheomykóznych dubov. Les 1985, 9, 391–394. [Google Scholar]
- Příhoda, A.; Jančařík, V. Tracheomykózy dubů. In TEI—Bulletin Technickoekonomických Informací 1; VÚLHM: Jíloviště-Strnady, Prague, Czech Republic, 1988; pp. 1–8. [Google Scholar]
- Kirisits, T. Studies on the Association of Ophiostomatoid Fungi with Bark Beetles in Austria with Special Emphasis on Ips typographus and Ips cembrae and Their Associated Fungi Ceratocystis polonica and Ceratocystis laricicola. Ph.D. Thesis, Universität für Bodenkultur (BOKU), Wien, Austria, 2001. [Google Scholar]
- Kirschner, R. Diversity of filamentous fungi in bark beetle galleries in central Europe. In Trichomycetes and Other Fungal Groups, 1st ed.; Lichtwardt Commemoration Volume; Robert, W., Misra, J.K., Horn, B.W., Eds.; Science Publishers, Inc.: Plymouth, UK, 2001; pp. 175–196. ISBN 9781578081325. [Google Scholar]
- Kotýnková-Sychrová, E. Mykoflóra chodeb kůrovců v Československu. Česká Mykol. 1966, 20, 45–53. [Google Scholar]
- Jankowiak, R. Fungi associated with Ips typographus on Picea abies in Southern Poland and their succession into the phloem and sapwood of beetle-infested trees and logs. For. Pathol. 2005, 35, 37–55. [Google Scholar] [CrossRef]
- Jankovský, L.; Mrkva, R. Přenos patogenů vaskulárních pletiv na smrku lýkožroutem smrkovým Ips typographus L. In Proceedings of the Les, Drevo, Životné Prostredie, Zbornik Referátov, Zvolen, Slovakia, 8–11 September 1997; Križová, E., Kodrík, M., Eds.; Technická Univerzita vo Zvolene: Zvolen, Slovakia, 1997; pp. 209–218. [Google Scholar]
- Żółciak, A. Occurrence of Armillaria species in oak stands in Poland. In Recent Advances on Oak Health in Europe, Proceedings of the International Scientific Conference, Warsaw, Poland, 22–24 November 1999; Oszako, T., Delatour, C., Eds.; Forest Research Institute: Warsaw, Poland, 2000; pp. 243–248. [Google Scholar]
- Kunca, A.; Leontovyč, R. Dieback of Broadleaved Trees in Slovakia. In Possible Limitation of Decline Phenomena in Broadleaved Stands; Oszako, T., Woodward, S.W., Eds.; Forest Research Institute: Warsaw, Poland, 2006; pp. 29–33. ISBN 83-87647-56-X. [Google Scholar]
- Szewczyk, W.; Kwaśna, H.; Behnke-Borowczyk, J. Armillaria Population in Flood-Plain Forest of Natural Pedunculate Oak Showing Oak Decline. Pol. J. Environ. Stud. 2016, 25, 1253–1262. [Google Scholar] [CrossRef] [Green Version]
- Mrkva, R. Praskliny kůry suchem jako poškození a dosud neznámý symptom chřadnutí listnatých dřevin. Zprávy Lesn. Výzkumu 2003, 48, 136–142. [Google Scholar]
- Jung, T.; Pérez-Sierra, A.; Durán, A.; Horta Jung, M.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil—And airborne Phytophthora species in forests and woodlands. Persoonia 2018, 40, 182–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, P.; Bader, M.K.-F.; Burgess, T.; Hardy, G.; Williams, N. Global biogeography and invasion risk of the plant pathogen genus Phytophthora. Environ. Sci. Policy 2019, 101, 175–182. [Google Scholar] [CrossRef]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Anguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef] [Green Version]
- Corcobado, T.; Solla, A.; Madeira, A.M.; Moreno, G. Combined effects of soil properties and Phytophthora cinnamomi infections on Quercus ilex decline. Plant Soil 2013, 373, 403–413. [Google Scholar] [CrossRef]
- Szabó, I.; Lakatos, F.; Sipos, G. Occurrence of soilborne Phytophthora species in declining broadleaf forest in Hungary. Eur. J. Plant Pathol. 2013, 137, 159–168. [Google Scholar] [CrossRef]
- Southwood, T.R.E. The number of species of insect associated with various trees. J. Anim. Ecol. 1961, 30, 1–8. [Google Scholar] [CrossRef]
- Knížek, M. Polník dvojtečný Agrilus biguttatus (Fabricius, 1777) (a ostatní krasci rodu Agrilus na dubech). Lesn. Práce 2011, 90, 1–4. [Google Scholar]
- Liška, J. Role Listožravého Hmyzu v Procesu Odumírání; Sub-final report to the project no. 329-91-9106; VÚLHM: Jíloviště-Strnady, Prague, Czech Republic, 1995. [Google Scholar]
- Jankovský, L.; Novotný, D.; Mrkva, R. Doprovodná mykoflóra. Ips typographus a ranové reakce smrku na umělou inokulaci imágy lýkožrouta smrkového. In Ochrana lesa a lesnická fytopatológia, Proceedings of the Conference, Zvolen, Sielnica, Slovakia, 4–6 September 2000; Hlaváč, P., Reinprecht, L., Gáper, J., Eds.; Technická Universita vo Zvolene: Zvolen, Slovakia, 2001. [Google Scholar]
- Kubátová, A.; Novotný, D.; Prášil, K. Microscopic fungi associated with oak bark beetle (Scolytus intricatus) in the Czech Rebublic. In IMC 7 Book of Abstracts, Proceedings of the 7th International Mycological Congress, Oslo, Norway, 11–17 August 2002; Universitat für Bodenkultur: Vienna, Austria; pp. 1–300.
- Šrůtka, P.; Pažoutová, S.; Kolařík, M. Daldinia decipiens and Entonaema cinnabarina as fungal symbionts of Xiphydria wood wasps. Mycol. Res. 2007, 111, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Soukup, F. Odumírání Dubů a Dalších Dřevin v Lesních Porostech s Příznaky Tracheomykózního Onemocnění; Final report to the project no. 329-91-9106; VÚLHM: Jíloviště-Strnady, Prague, Czech Republic, 1995. [Google Scholar]
- Sallé, A.; Nageleisen, L.M.; Lieutier, F. Bark and wood boring insects involved in oak declines in Europe: Current knowledge and future prospects in a context of climate change. For. Ecol. Manag. 2014, 328, 79–93. [Google Scholar] [CrossRef]
- Czech Hydrometeorological Institute (CHMI). Historical Data—Meteorology and Climatology. Daily Data. Available online: https://www.chmi.cz/historicka-data/pocasi/denni-data/Denni-data-dle-z.-123-1998-Sb (accessed on 1 June 2022).
- Harris, I.C.; Jones, P.D.; Osborn, T. CRU TS4.05: Climatic Research Unit (CRU) Time-Series (TS) Version 4.05 of High-Resolution Gridded Data of Month-by-Month Variation in Climate (January 1901–December 2020); University of East Anglia Climatic Research Unit; NERC EDS Centre for Environmental Data Analysis: Norwich, UK, 2021. [Google Scholar]
- Gregus, L.; Heško, J.; Hruška, J.; Jančařík, V.; Mráček, Z.; Pokorný, J.; Potoček, J.; Příhoda, A.; Zumr, V. Záchrana Jilmů—Metodická Příručka č. 9; ÚV Českého Svazu Ochránců Přírody: Prague, Czech Republic, 1986; pp. 1–46. [Google Scholar]
- Jung, T.; Blaschke, M. Phytophthora root and collar rot of alders in Bavaria: Distribution, modes of spread and possible management strategies. Plant Pathol. 2004, 53, 197–208. [Google Scholar] [CrossRef]
- Jung, T.; Burgess, T.I. Re-evaluation of Phytophthora citricola isolates from multiple woody hosts in Europe and North America reveals a new species, Phytophthora plurivora sp. nov. Persoonia 2009, 22, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; The American Phytopathological Society Press: St Paul, MN, USA, 1996; ISBN 0-89054-212-0. [Google Scholar]
- Grünwald, N.J.; Martin, F.N.; Larsen, M.M.S.; Press, C.M.; Coffey, M.D.; Hansen, E.M.; Parke, J.L. Phytophthora-ID.org: A sequence-based Phytophthora identification tool. Plant Dis. 2011, 95, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vansteenkiste, D.; Tirry, L.; Van Acker, J.; Stevens, M. Predispositions and symptoms of Agrilus borer attack in declining oak trees. Ann. For. Sci. 2004, 61, 815–823. [Google Scholar] [CrossRef] [Green Version]
- Pilcher, J.R.; Gray, B. The relationships between oak tree growth and climate in Britain. J. Ecol. 1982, 70, 297–304. [Google Scholar] [CrossRef]
- Thomas, F.M.; Hartmann, G. Tree rooting patterns and soil water relations of healthy 4 and damaged stands of mature oak (Quercus robur L. and Quercus petraea [Matt.] Liebl.). Plant Soil 1998, 203, 145–158. [Google Scholar] [CrossRef]
- Bréda, N. Water shortage as a key factor in the case of the oak dieback in the Harth Forest (Alsatian plain, France) as demonstrated by dendroecological and ecophysiological study. In Recent Advances on Oak Health in Europe, Proceedings of the International Scientific Conference, Warsaw, Poland, 22–24 November 1999; Oszako, T., Delatour, C., Eds.; Forest Research Institute: Warsaw, Poland, 2000; pp. 157–159. [Google Scholar]
- Wermelinger, B.; Rigling, A.; Schneider, M.D.; Dobbertin, M. Assessing the role of bark-and wood-boring insects in the decline of Scots pine (Pinus sylvestris) in the Swiss Rhone valley. Ecol. Entomol. 2008, 33, 239–249. [Google Scholar] [CrossRef]
- Leontovyč, R.; Kunca, A. Health condition of oak stands in Slovakia. In Recent Advances on Oak Health in Europe, Proceedings of the International Scientific Conference, Warsaw, Poland, 22–24 November 1999; Oszako, T., Delatour, C., Eds.; Forest Research Institute: Warsaw, Poland, 2000; pp. 105–106. [Google Scholar]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Leontovyč, R. Výskyt rodu Ophiostoma na listnatých dřevinách v súčasných zmenených ekologických podmienkach Slovenska. In Proceedings of the Ophiostomatales—Výsledky současného taxonomického a fytopatologického výzkumu. Sborník referátů, Praha, Czech Republic, 29 May 1991; Holubová, V., Prášil, K., Eds.; Československá Vědecká Společnost pro Mykologii při ČSAV: Prague, Czech Republic, 1992; pp. 30–35. [Google Scholar]
- McIntyre, G.A.; Jacobi, W.R.; Ramaley, A.W. Factors affecting cytospora canker occurrence on aspen. J. Arboric. 1996, 22, 229–233. [Google Scholar] [CrossRef]
- Haavik, L.J.; Stephen, F.M. Factors that affect compartmentalization and wound closure of Quercus rubra infested with Enaphalodes rufulus. Agric. For. Entomol 2011, 13, 291–300. [Google Scholar] [CrossRef]
- Sierpinski, A.; Hilszczański, J. Agrilus spp. The Main Factor of Oak Decline In Poland. In Possible Limitation of Decline Phenomena in Broadleaved Stands; Oszako, T., Woodward, S.W., Eds.; Forest Research Institute: Warsaw, Poland, 2006; pp. 159–161. ISBN 83-87647-56-X. [Google Scholar]
- Drobyshev, I.; Linderson, H.; Sonesson, K. Temporal mortality pattern of pedunculate oaks in southern Sweden. Dendrochronologia 2007, 24, 97–108. [Google Scholar] [CrossRef]
- Evans, H.F.; Moraal, L.; Pajares, J.A. Biology, ecology and economic importance of Buprestidae and Cerambycidae. In European Bark and Wood Boring Insects in Living Trees: A Synthesis; Lieutier, F., Day, K., Battisti, A., Gregoire, J.C., Evans, H., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 447–474. ISBN 978-1-4020-2240-1. [Google Scholar]
- Brown, N.; Inward, D.J.G.; Jeger, M.; Denman, S. A Review of Agrilus Biguttatus in UK Forests and Its Relationship with Acute Oak Decline. Forestry 2015, 88, 53–63. [Google Scholar] [CrossRef]
- Denman, S.; Kirk, S.A.; Webber, J.F. Managing Acute Oak Decline; Forestry Commission: Farnham, UK, 2010; 6p, ISBN 978-0-85538-802-7. [Google Scholar]
- Knížek, M. Bělokaz dubový. Scolytus intricatus (Ratzeburg). Lesn. Práce 2002, 81, 12, Annex I–Annex IV. [Google Scholar]
- Patočka, J.; Novotný, J. Účasť hmyzu na hromadnom hynutí dubov na Slovensku. In Problematika Hynutia Dubov na Slovensku, Vedecké Práce VÚLH vo Zvolene; Príroda v Bratislave: Bratislava, Slovakia, 1987; Volume 36, pp. 59–90. [Google Scholar]
- Gogola, E.; Chovanec, D. Podkornik Dubový a Tracheomykóza Dubov; Videopress MON: Bratislava, Slovakia, 1987. [Google Scholar]
- Farkač, J.; Král, D.; Škorpík, M. Červený Seznam Ohrožených Druhů České Republiky—Bezobratlí; Agentura ochrany přírody a krajiny ČR: Prague, Czech Republic, 2005; ISBN 80-86064-96-4. [Google Scholar]
- Hejda, R.; Farkač, J.; Chobot, K. Červený Seznam Ohrožených Druhů České republiky—Bezobratlí; Agentura ochrany přírody a krajiny ČR: Prague, Czech Republic, 2017; ISBN 978-80-88076-53-7. [Google Scholar]
- Głowaciński, Z.; Nowacki, J. Polska Czerwona Księga Zwierząt—Bezkręgowce; Instytut Ochrony Przyrody PAN: Kraków, Poland, 2004; ISBN 83-88934-60-0. [Google Scholar]
- Binot, M.; Bless, R.; Boye, P.; Gruttke, H.; Pretscher, P. Rote Liste Gefährdeter Tiere Deutschlands; Bundesamt für Naturschutz: Leipzig, Germany, 1998; ISBN 3-89624-110-9. [Google Scholar]
- Denman, S.; Webber, J. Oak declines: New definitions and new episodes in Britain. Q. J. For. 2009, 103, 285–290. [Google Scholar]
- Brady, C.; Denman, S.; Kirk, S.; Venter, S.; Rodriguez-Palenzuela, P.; Coutinho, T. Description of Gibbsiella quercinecans gen. nov., sp. nov., associated with acute oak decline. Syst. Appl. Microbiol. 2010, 33, 444–450. [Google Scholar] [CrossRef]
- Denman, S.; Brady, C.; Kirk, S.; Cleenwerck, I.; Venter, S.; Countinho, T.; De Vos, P. Brenneria goodwinii sp. Nov., Associated with Acute Oak Decline in the UK. Int. J. Syst. Evol. Microbiol. 2012, 62, 2451–2456. [Google Scholar] [CrossRef]
- Szontagh, P. Die Rolle der Insektengradation im Verlauf der Krankheiten von Trauben-eichenbeständen. Osterreichische Forstztg. 1987, 98, 65–66. [Google Scholar]
- Schopf, A. Rinden-und holzbriitende Schädlinge an erkrankten Eichen in Österreich. Osterreichische Forstztg. 1992, 103, 33–35. [Google Scholar]
- Mrázková, M.; Černý, K.; Tomšovský, M.; Holub, V.; Strnadová, V.; Zlatohlávek, A.; Gabrielová, S. First Report of Root Rot of Pedunculate Oak and Other Forest Tree Species Caused by Phytophthora plurivora in the Czech Republic. Plant Dis. 2010, 94, 2. [Google Scholar] [CrossRef] [PubMed]
- Černý, K.; Tomšovský, M.; Mrázková, M.; Strnadová, V. The present state of knowledge of Phytophthora spp. Diversity in forest and ornamental woody plants in the Czech Republic. N. Z. J. For. Sci. 2011, 41, S75–S82. [Google Scholar]
- Mrázková, M.; Černý, K.; Strnadová, V.; Filipová, N. Identifikace Symptomů Napadení Dřevin a Okrasných Rostlin Patogeny z rodu Phytophthora de Bary. Certifikovaná metodika; VÚKOZ: Průhonice, Czech Republic, 2012. [Google Scholar]
- Mrázková, M.; Černý, K.; Tomšovský, M.; Strnadová, V.; Gregorová, B.; Holub, V.; Pánek, M.; Havrdová, L.; Hejná, M. Occurrence of Phytophthora multivora and Phytophthora plurivora in the Czech Republic. Plant Protect. Sci. 2013, 49, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Jankowiak, R.; Stępniewska, H.; Bilański, P.; Kolařík, M. Occurrence of Phytophthora Plurivora and Other Phytophthora Species in Oak Forests of Southern Poland and Their Association with Site Conditions and the Health Status of Trees. Folia Microbiol. 2014, 59, 531–542. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Sikora, K.; Galko, J.; Kunca, A.; Milenković, I. Isolation and Pathogenicity of Phytophthora Species from Sessile Oak (Quercus Petraea (Matt.) Liebl.) Stands in Slovakia. For. Pathol. 2020, 50, 330. [Google Scholar] [CrossRef]
- Milenković, I.; Keča, N.; Karadžić, D.; Radulović, Z.; Nowakovska, J.A.; Oszako, T.; Sikora, K.; Corcobado, T.; Jung, T. Isolation and Pathogenicity of Phytophthora Species from Poplar Plantations in Serbia. Forests 2018, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Jančařík, V. Fytopatologické problémy působené houbami rodu Ophiostoma a možnosti ochrany. In Proceedings of the Ophiostomatales—Výsledky Současného Taxonomického a Fytopatologického Výzkumu. Sborník Referátů, Prague, Czech Republic, 29 May 1991; Holubová, V., Prášil, K., Eds.; Československá Vědecká Společnost pro Mykologii při ČSAV: Prague, Czech Republic, 1992; pp. 95–110. [Google Scholar]
- Čížková, D.; Švecová, M. Gradual oaks decline with tracheomycotic symptoms in green areas outside forests in Southern Bohemia. In Aktuální problémy ochrany dřevin. Sborník referátů, Prachatice, Czech Republic, 3–4 October 1995; Čížková, D., Švecová, M., Eds.; Ministerstvo Životního Prostředí ČR: Prague, Czech Republic, 1995; Volume 1, pp. 18–23. [Google Scholar]
- Dominik, J.; Starczyk, J.R. Owady Niszczące Drewno; Panstwowe Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 1988. [Google Scholar]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef]






| Study Plot | Latitude; Longitude | Altitude (m a.s.l.) | Age | Average DBH (cm) | Average Height (m) |
|---|---|---|---|---|---|
| Pístecký les | 50°25′25.3″ N 14°08′2.7″ E | 198 | 60 | 31.4 | 26.8 |
| Tvořihráz | 48°54′27.5″ N 16°06′31.6″ E | 310 | 38 | 31 | 19.6 |
| Česká Skalice | 50°22′55.2″ N 16°02′27.2″ E | 318 | 40 | 19 | 19.5 |
| Nižbor | 50°00′09.9″ N 13°57′50.4″ E | 351 | 48 | 14.4 | 12.3 |
| Český kras | 49°56′50.3″ N 14°08′41.0″ E | 363 | 42 | 18.9 | 18.5 |
| Lásenice | 49°02′14.2″ N 14°58′54.3″ E | 530 | 110 | 36 | 29.2 |
| Locality | Tree Part | Intensity (0–3) |
|---|---|---|
| Pístecký les | basal | 0 |
| middle | 1 | |
| crown | 1 | |
| Tvořihráz | basal | 1 |
| middle | 3 | |
| crown | 1 | |
| Česká Skalice | basal | 2 |
| middle | 1 | |
| crown | 1 | |
| Nižbor | basal | 3 |
| middle | 1 | |
| crown | 0 | |
| Český kras | basal | 0 |
| middle | 0 | |
| crown | 3 | |
| Lásenice | basal | 3 |
| middle | 1 | |
| crown | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macháčová, M.; Nakládal, O.; Samek, M.; Baťa, D.; Zumr, V.; Pešková, V. Oak Decline Caused by Biotic and Abiotic Factors in Central Europe: A Case Study from the Czech Republic. Forests 2022, 13, 1223. https://doi.org/10.3390/f13081223
Macháčová M, Nakládal O, Samek M, Baťa D, Zumr V, Pešková V. Oak Decline Caused by Biotic and Abiotic Factors in Central Europe: A Case Study from the Czech Republic. Forests. 2022; 13(8):1223. https://doi.org/10.3390/f13081223
Chicago/Turabian StyleMacháčová, Markéta, Oto Nakládal, Michal Samek, Daniel Baťa, Václav Zumr, and Vítězslava Pešková. 2022. "Oak Decline Caused by Biotic and Abiotic Factors in Central Europe: A Case Study from the Czech Republic" Forests 13, no. 8: 1223. https://doi.org/10.3390/f13081223
APA StyleMacháčová, M., Nakládal, O., Samek, M., Baťa, D., Zumr, V., & Pešková, V. (2022). Oak Decline Caused by Biotic and Abiotic Factors in Central Europe: A Case Study from the Czech Republic. Forests, 13(8), 1223. https://doi.org/10.3390/f13081223