Biological Activities in Sapwood and Heartwood Extractives from Paulownia tomentosa
Abstract
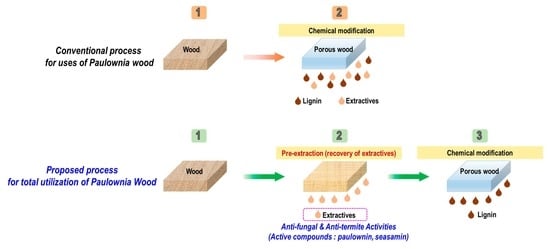
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Decay Resistance Test
2.2.1. Sample Preparation
2.2.2. Fungal Culture
2.2.3. Decay Resistance Test
2.2.4. Scanning Electronic Microscopy
2.3. Termite Resistance Test
2.4. Anti-Fungal and Anti-Oxidant Activities
2.4.1. Preparation of Crude Extract and Solvent Fractionation
2.4.2. Thin-Layer Chromatography
2.4.3. Anti-Fungal Activity
2.4.4. Anti-Oxidant Activity
- 2,2-Diphenyl-1-picrylhydrazyl(DPPH) radical-scavenging activity
- 2.
- 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity
2.4.5. Quantitative and Qualitative Analyses
2.5. Statistical Analysis
3. Results and Discussion
3.1. Decay Resistance Test
3.1.1. Weight Loss
3.1.2. Cell Wall Changes
3.2. Termite Resistance Test
3.2.1. Weight Loss
3.2.2. Mortality
3.3. Evaluation of Biological Activities
3.3.1. Crude Extract and Solvent Fraction Yield
3.3.2. Extract Distribution Using TLC
3.3.3. Anti-Fungal Activity
3.3.4. Anti-Oxidant Activities
3.3.5. Quantitative and Qualitative Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perré, P.; Huber, F. Measurement of free shrinkage at the tissue level using an optical microscope with an immersion objective: Results obtained for Douglas fir (Pseudotsuga menziesii) and spruce (Picea abies). Ann. For. Sci. 2007, 64, 255–265. [Google Scholar] [CrossRef]
- Su, N.Y.; Scheffrahn, R.H. Termites as Pests of Buildings. In Termites: Evolution, Sociality, Symbioses, Ecology; Springer: Dordrecht, The Netherlands, 2000; pp. 437–453. [Google Scholar]
- Procópio, L.; Barreto, C. The soil microbiomes of the Brazilian Cerrado. J. Soils Sediments 2021, 21, 2327–2342. [Google Scholar] [CrossRef]
- Blanchette, R.A. Delignification by wood-decay fungi. Annu. Rev. Phytopathol. 1991, 29, 381–403. [Google Scholar] [CrossRef]
- Kim, Y.S. Wood Conservation Science; Chonnam National University Press: Gwangju, Republic of Korea, 2019; Volume 53. [Google Scholar]
- Hill, C.A.; Forster, S.C.; Farahani, M.R.M.; Hale, M.D.C.; Ormondroyd, G.A.; Williams, G.R. An investigation of cell wall micropore blocking as a possible mechanism for the decay resistance of anhydride modified wood. Int. Biodeterior. Biodegrad. 2005, 55, 69–76. [Google Scholar] [CrossRef]
- Khakalo, A.; Tanaka, A.; Korpela, A.; Orelma, H. Delignification and ionic liquid treatment of wood toward multifunctional high-performance structural materials. ACS Appl. Mater. Interfaces 2020, 12, 23532–23542. [Google Scholar] [CrossRef] [PubMed]
- Vidholdová, Z.; Kačík, F.; Reinprecht, L.; Kučerová, V.; Luptáková, J. Changes in chemical structure of thermally modified spruce wood due to decaying fungi. J. Fungi 2022, 8, 739. [Google Scholar] [CrossRef]
- Montanari, C.; Ogawa, Y.; Olsén, P.; Berglund, L.A. High performance, fully bio-based, and optically transparent wood biocomposites. Adv. Sci. 2021, 8, 2100559. [Google Scholar] [CrossRef]
- Qin, J.; Li, X.; Shao, Y.; Shi, K.; Zhao, X.; Feng, T.; Hu, Y. Optimisation of delignification process for efficient preparation of transparent wood with high strength and high transmittance. Vacuum 2018, 158, 158–165. [Google Scholar] [CrossRef]
- Li, H.; Guo, X.; He, Y.; Zheng, R. A green steam-modified delignification method to prepare low-lignin delignified wood for thick, large highly transparent wood composites. J. Mater. Res. 2019, 34, 932–940. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Q.; Rojas, R.; Yan, M.; Lawoko, M.; Berglund, L. Lignin-retaining transparent wood. ChemSusChem 2017, 10, 3445–3451. [Google Scholar] [CrossRef]
- Park, K.; Kim, B.; Park, H.; Kim, Y.; Park, S. Characterisation of a translucent material produced from Paulownia tomentosa using peracetic acid delignification and resin infiltration. Polymers 2022, 14, 4380. [Google Scholar] [CrossRef] [PubMed]
- Icka, P.; Damo, R.; Icka, E. Paulownia tomentosa, a fast growing timber. Annals of “Valahia” University of Târgovişte. Agriculture 2016, 10, 14–19. [Google Scholar]
- Jung, S.H.; Park, B.S. Wood Properties of the Useful Tree Species Grown; Korea Forest Research Institute: Seoul, Republic of Korea, 2008; Volume 29. [Google Scholar]
- Sánchez-Machado, J.D.; Moya, R. Characteriztion of Paulownia tomentosa steud trees grown in a 5-year-old plantation in Costa Rica. Cellul. Chem. Technol. 2021, 55, 743–753. [Google Scholar] [CrossRef]
- Kawamura, F.; Ohara, S.; Nishida, A. Anti-fungal activity of constituents from the heartwood of Gmelina arborea: Part 1. Sensitive anti-fungal assay against Basidiomycetes. Holzforschung 2004, 58, 189–192. [Google Scholar] [CrossRef]
- Kim, T.; Min, K.; Yu, S.; Lee, M.; Jung, H.; Cho, W.; Kim, M.; Chun, W.; Kwon, Y. Chemical constituents of the twigs of Paulownia coreana. Korean J. Pharmacogn. 2015, 46, 99–104. [Google Scholar]
- Park, Y.M.; Ki, J.S.; Kim, Y.S.; Kim, B.K. The Constituents of Paulownia tomentosa stem. Yakkak Hoeji 1991, 35, 301–307. [Google Scholar]
- KS F 2213; Laboratory Test Method of Natural Decay Resistance of Wood. DKorean Standards & Certification: Seoul, Republic of Korea, 2018.
- Li, Q.; Wang, X.; Lin, J.; Liu, J.; Jiang, M.; Chu, L. Chemical Composition and Anti-fungal Activity of Extracts from the Xylem of Cinnamomum camphora. Bioresources 2014, 9, 2560–2571. [Google Scholar]
- Lim, J.; Choi, Y.; Jung, M.; Kang, S.; Chung, Y. Anti-fungal and insecticidal activity of methanol extract from 11 Korean wood species. J. Conserv. Sci. 2008, 23, 95–102. [Google Scholar]
- Kirker, G.T.; Blodgett, A.B.; Arango, R.A.; Lebow, P.K.; Clausen, C.A. The role of extractives in naturally durable wood species. Int. Biodeterior. Biodegrad. 2013, 82, 53–58. [Google Scholar] [CrossRef]
- Zabel, R.; Morrell, J. Wood Microbiology, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Kim, Y. Evaluation of Termite Resistance and Weather Resistance of Paulownia Wood Materials. Master’s Thesis, Kangwon National University Graduate School, Gangwon, Republic of Korea, 2018. [Google Scholar]
- JIS K 1571; Test Methods for Determining the Effectiveness of Wood Preservatives and Their Performance Requirements. Japanese Standards Association: Tokyo, Japan, 2004.
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Waster & Grugter: New York, NY, USA, 1984; p. 613. [Google Scholar]
- Harju, A.; Venäläinen, M.; Anttonen, S.; Viitanen, H.A.; Kainulainen, P.S.; Saranpää, P.; Vapaavuori, E. Chemical factors affecting the brown-rot decay resistance of Scots pine heartwood. Trees 2003, 17, 263–268. [Google Scholar] [CrossRef]
- Lee, K.; Jung, S.Y. Ecological characteristics of termite (Reticulitermes speratus kyushuensis) for preservation of wooden cultural heritage. MUNHWAJAE Korean J. Cult. Herit. Stud. 2004, 37, 327–348. [Google Scholar]
- Esteves, B.; Ferreira, H.; Viana, H.; Ferreira, J.; Domingos, I.; Cruz-Lopes, L.P.; Jones, D.; Nunes, L. Termite resistance, chemical and mechanical characterization of Paulownia tomentosa wood before and after heat treatment. Forests 2021, 12, 1114. [Google Scholar] [CrossRef]
- Tabassam, Q.; Mehmood, T.; Ahmed, S.; Saeed, S.; Raza, A.R.; Anwar, F. GC-MS Metabolomics profiling and HR-APCI-MS characterisation of potential anticancer compounds and anti-microbial activities of extracts from Picrorhiza kurroa roots. J. Appl. Biomed. 2021, 19, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Jameel, S.; Ahmad, S.; Akram, M.; Zainab, R.; Sharif, A. Anti-fungal activity in the methanolic, aqueous and hexane extracts of Capparis deciduas. Eur. J. Inflamm. 2018, 16, 2058739218781701. [Google Scholar] [CrossRef]
- Si, C.; Kim, J.; Gwon, D.; Bae, Y. Phenolic compounds from the fruits of Paulownia coreana uyeki. J. Korean Wood Sci. Technol. 2006, 34, 79–85. [Google Scholar]
- Meng, Z.F.; Guo, X.F.; Zhu, Y.; Jing, S.K. Analysis of anti-oxidant properties and major components of the extract of Paulownia tomentosa steud flowers. Adv. Mater. Res. 2014, 1010–1012, 164–177. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Chao, C.; Lu, X.; Xiong, Y.G. Paulownia in China: Cultivation and Utilisation; International Development Research Centre: Ottawa, ON, Canada, 1986. [Google Scholar]








| Fomitopsis palustris | Trametes versicolor | |
|---|---|---|
| Control—heartwood | 28.77 ± 3.25 | 12.37 ± 3.64 * |
| Control—sapwood | 16.63 ± 1.54 * | 31.75 ± 6.26 |
| Extracted—heartwood | 44.93 ± 16.66 | 31.52 ± 2.65 * |
| Extracted—sapwood | 53.54 ± 4.53 * | 35.51 ± 10.58 |
| Average Weight Loss (%) | Class of Resistance |
|---|---|
| 0–10 | Highly resistant |
| 11–24 | Resistant |
| 25–44 | Moderately resistant |
| >45 | Slightly resistant or nonresistant |
| Weight Loss Rate | Mortality | |
|---|---|---|
| Control—heartwood | 12.6 ± 2.27 * | 16.3 ± 6.17 * |
| Control—sapwood | 9.38 ± 0.80 * | 24 ± 1.77 * |
| Extracted—heartwood | 45.95 ± 4.17 * | 2.10 ± 3.13 * |
| Extracted—sapwood | 31.00 ± 1.27 * | 6.20 ± 2.91 * |
| Sapwood | Heartwood | |
|---|---|---|
| Crude (Total extractives) | 8.43 ± 0.05 | 7.59 ± 0.63 |
| Hexane | 4.03 ± 0.02 | 2.80 ± 0.36 |
| Chloroform | 15.57 ± 1.70 | 12.92 ± 3.51 |
| Ethyl acetate | 11.61 ± 0.92 | 9.74 ± 1.56 |
| Water | 50.68 ± 2.09 | 58.70 ± 6.68 |
| Residue * | 18.12 | 15.85 |
| Concentration (ppm) | T. versicolor | F. palustris | |||
|---|---|---|---|---|---|
| Sapwood | Heartwood | Sapwood | Heartwood | ||
| Crude | 0 | 40 | 40 | 45 | 35 |
| 10,000 | 40 | 40 | 40 | 35 | |
| 50,000 | 35 | 40 | 40 | 35 | |
| 100,000 | 25 | 40 | 35 | 35 | |
| Hexane | 0 | 40 | 45 | 40 | 35 |
| 10,000 | 40 | 38 | 35 | 30 | |
| 50,000 | 30 | 30 | 25 | 30 | |
| 100,000 | 25 | 20 | 25 | 18 | |
| Chloroform | 0 | 40 | 40 | 45 | 40 |
| 10,000 | 35 | 38 | 35 | 38 | |
| 50,000 | 30 | 30 | 30 | 30 | |
| 100,000 | 20 | 26 | 26 | 25 | |
| Ethyl acetate | 0 | 40 | 45 | 45 | 40 |
| 10,000 | 40 | 45 | 45 | 40 | |
| 50,000 | 35 | 40 | 45 | 40 | |
| 100,000 | 35 | 30 | 40 | 35 | |
| Water | 0 | 35 | 40 | 35 | 40 |
| 10,000 | 35 | 40 | 35 | 40 | |
| 50,000 | 35 | 40 | 35 | 40 | |
| 100,000 | 35 | 40 | 35 | 40 | |
| Concentration (ppm) | T. harzianum | A. niger | P. glabrum | ||||
|---|---|---|---|---|---|---|---|
| Sapwood | Heartwood | Sapwood | Heartwood | Sapwood | Heartwood | ||
| Crude | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10,000 | 0 | 0 | 0 | 0 | 0 | 13 | |
| 50,000 | 0 | 15 | 0 | 0 | 0 | 15 | |
| 100,000 | 0 | 20 | 0 | 0 | 0 | 15 | |
| Hexane | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10,000 | 30 | 20 | 11 | 0 | 15 | 13 | |
| 50,000 | 30 | 30 | 11 | 0 | 20 | 15 | |
| 100,000 | 30 | 30 | 11 | 0 | 20 | 20 | |
| Chloroform | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10,000 | 25 | 25 | 0 | 0 | 15 | 14 | |
| 50,000 | 30 | 30 | 0 | 0 | 15 | 15 | |
| 100,000 | 30 | 30 | 0 | 0 | 20 | 20 | |
| Ethyl acetate | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10,000 | 0 | 0 | 11 | 0 | 0 | 0 | |
| 50,000 | 0 | 0 | 11 | 0 | 0 | 0 | |
| 100,000 | 0 | 0 | 11 | 0 | 0 | 0 | |
| Water | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10,000 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 50,000 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 100,000 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Sample | IC50 * (µg/mL) | ||
|---|---|---|---|
| DPPH Radical-Scavenging Activity | ABTS Radical-Scavenging Activity | ||
| P. tomentosa Heartwood | Crude | 37.84 | 18.00 |
| Hexane | 130.95 | 507.43 | |
| Chloroform | 95.63 | 30.42 | |
| Ethyl acetate | 25.39 | 8.61 | |
| Water | 27.83 | 17.24 | |
| P. tomentosa Sapwood | Crude | 55.78 | 15.36 |
| Hexane | 90.42 | 418.43 | |
| Chloroform | 86.19 | 17.63 | |
| Ethyl acetate | 24.93 | 8.11 | |
| Water | 42.09 | 15.69 | |
| Ascorbic acid (Standard compounds) | 6.01 | 5.78 | |
| Part | Sample | Sesamin | Paulownin |
|---|---|---|---|
| Content (µg/mL) | Content (µg/mL) | ||
| Sapwood | Crude | 13.94 | 25.71 |
| Hexane | 37.60 | 63.69 | |
| Chloroform | 17.96 | 68.23 | |
| Heartwood | Crude | 13.00 | 25.68 |
| Hexane | 44.92 | 66.71 | |
| Chloroform | 19.20 | 62.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Kim, B.; Park, K.-C.; Kim, Y.; Kim, T.; Kim, M.-S.; Choi, S.-E.; Park, S.-Y. Biological Activities in Sapwood and Heartwood Extractives from Paulownia tomentosa. Forests 2023, 14, 2171. https://doi.org/10.3390/f14112171
Park H, Kim B, Park K-C, Kim Y, Kim T, Kim M-S, Choi S-E, Park S-Y. Biological Activities in Sapwood and Heartwood Extractives from Paulownia tomentosa. Forests. 2023; 14(11):2171. https://doi.org/10.3390/f14112171
Chicago/Turabian StylePark, Hanna, Byeongho Kim, Kyoung-Chan Park, Yesun Kim, Taehee Kim, Min-Seok Kim, Sun-Eun Choi, and Se-Yeong Park. 2023. "Biological Activities in Sapwood and Heartwood Extractives from Paulownia tomentosa" Forests 14, no. 11: 2171. https://doi.org/10.3390/f14112171
APA StylePark, H., Kim, B., Park, K. -C., Kim, Y., Kim, T., Kim, M. -S., Choi, S. -E., & Park, S. -Y. (2023). Biological Activities in Sapwood and Heartwood Extractives from Paulownia tomentosa. Forests, 14(11), 2171. https://doi.org/10.3390/f14112171