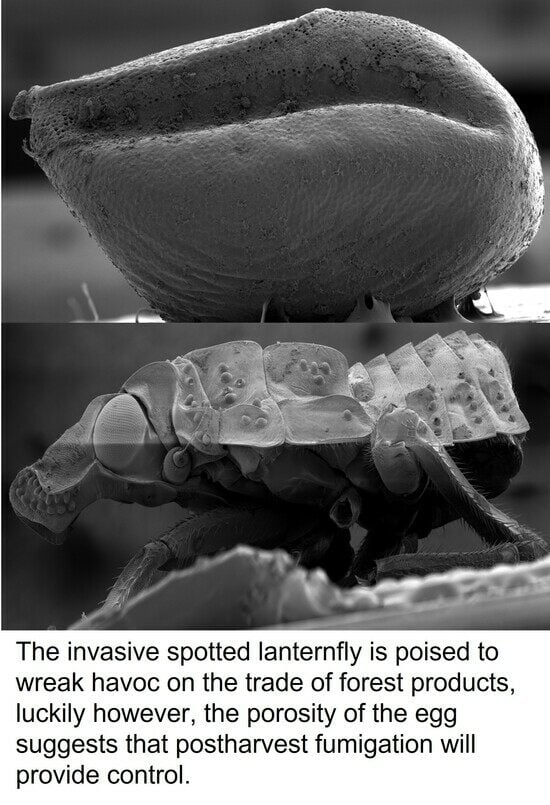
Egg Morphology and Chorionic Ultrastructure of Spotted Lanternfly, Lycorma delicatula (White) (Hemiptera: Fulgoridae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Egg Collection
2.2. Microscopy
2.2.1. Scanning Electron Microscopy
2.2.2. Time-Lapse Light Microscopy
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barringer, L.E.; Donovall, L.R.; Spichiger, S.-E.; Lynch, D.; Henry, D. The first new world record of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae). Entomol. News 2015, 125, 20–23. [Google Scholar] [CrossRef]
- Dara, S.K.; Barringer, L.; Arthurs, S.P. Lycorma delicatula (Hemiptera: Fulgoridae): A new invasive pest in the United States. J. Integr. Pest Manag. 2015, 6, 20. [Google Scholar] [CrossRef]
- Center, N.I. Spotted Laternfly Map. Available online: https://www.stopslf.org/where-is-slf/slf-map/ (accessed on 22 November 2023).
- Han, J.M.; Kim, H.; Lim, E.J.; Lee, S.; Kwon, Y.J.; Cho, S. Lycorma delicatula (Hemiptera: Auchenorrhyncha: Fulgoridae: Aphaeninae) finally, but suddenly arrived in Korea. Entomol. Res. 2008, 38, 281–286. [Google Scholar] [CrossRef]
- Lee, D.-H.; Park, Y.-L.; Leskey, T.C. A review of biology and management of Lycorma delicatula (Hemiptera: Fulgoridae), an emerging global invasive species. J. Asia-Pac. Entomol. 2019, 22, 589–596. [Google Scholar] [CrossRef]
- Barringer, L.; Ciafré, C.M. Worldwide feeding host plants of spotted lanternfly, with significant additions from North America. Environ. Entomol. 2020, 49, 999–1011. [Google Scholar] [CrossRef]
- Liu, H. Oviposition substrate selection, egg mass characteristics, host preference, and life history of the spotted lanternfly (Hemiptera: Fulgoridae) in North America. Environ. Entomol. 2019, 48, 1452–1468. [Google Scholar] [CrossRef]
- Cheetham, T. Pathological Alterations in Embryos of the Codling Moth (Lepidoptera: Tortricidae) Induced by Methyl Bromide. Ann. Entomol. Soc. Am. 1990, 83, 59–67. [Google Scholar] [CrossRef]
- Mostafa, S.; Kamel, A.; El-Nahal, A.; El-Borollosy, F. Toxicity of carbon bisulphide and methyl bromide to the eggs of four stored product insects. J. Stored Prod. Res. 1972, 8, 193–198. [Google Scholar] [CrossRef]
- Nitschke, K.; Albee, R.; Mattsson, J.; Miller, R. Incapacitation and treatment of rats exposed to a lethal dose of sulfuryl fluoride. Fundam. Appl. Toxicol. 1986, 7, 664–670. [Google Scholar] [CrossRef]
- Mendrala, A.; Markham, D.; Eisenbrandt, D. Rapid uptake, metabolism, and elimination of inhaled sulfuryl fluoride fumigant by rats. Toxicol. Sci. 2005, 86, 239–247. [Google Scholar] [CrossRef]
- Meikle, R.; Stewart, D.; Globus, O. Fumigant mode of action, drywood termite metabolism of Vikane fumigant as shown by labeled pool technique. J. Agric. Food Chem. 1963, 11, 226–230. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Y.H.; Kim, G.; Lee, B.-H.; Yang, J.-O.; Lee, S.-E. Ethyl formate and phosphine fumigations on the two-spotted spider mite, Tetranychus urticae and their biochemical responses. Appl. Biol. Chem. 2019, 62, 50. [Google Scholar] [CrossRef]
- Haritos, V.; Dojchinov, G. Cytochrome c oxidase inhibition in the rice weevil Sitophilus oryzae (L.) by formate, the toxic metabolite of volatile alkyl formates. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, P. Formate as an inhibitor of cytochrome c oxidase. Biochem. Biophys. Res. Commun. 1975, 67, 610–616. [Google Scholar] [CrossRef]
- Kim, K.; Lee, B.-H.; Park, J.S.; Yang, J.O.; Lee, S.-E. Biochemical mechanisms of fumigant toxicity by ethyl formate towards Myzus persicae nymphs. J. Appl. Biol. Chem. 2017, 60, 271–277. [Google Scholar] [CrossRef]
- FAO. International Standards for Phytosanitary Measures (ISPM) No. 28. In Phytosanitary Treatments for Regulated Pests; FAO: Rome, Italy, 2007; pp. 3–11. [Google Scholar]
- United Nations Environment Program. Report of the Methyl Bromide Technical Options Committee (MBTOC)—2010 Assessment; UNEP: Nariobi, Kenya, 2010.
- Kenaga, E. Some biological, chemical and physical properties of sulfuryl fluoride as an insecticidal fumigant. J. Econ. Entomol. 1957, 50, 1–6. [Google Scholar] [CrossRef]
- Reichmuth, C.S.M.; Dugast, J.-F.; Drinkall, M.J. On the efficacy of sulphuryl fluoride against stored product pest moths and beetles. In Proceedings of the Conference on Controlled Atmosphere and Fumigation in Stored Products, Nicosia, Cyprus, 21–26 April 1997. [Google Scholar]
- United Nations Environment Program. Special Review on Achieving Control of Pest Eggs by Sulfuryl Fluoride; Report of the Technology and Economic Assessment Panel; UNEP: Nairobi, Kenya, 2011; pp. 110–136.
- Walse, S.S.; Gautam, S.G.; Opit, G.P.; Margosan, D.; Tebbets, J.S. Sulfuryl fluoride-propylene oxide mixtures: Applications and efficacy. In Proceedings of the International Working Congress on Stored Product Protection, Chiang Mai, Thailand, 23 November 2014; Available online: http://spiru.cgahr.ksu.edu/proj/iwcspp/iwcspp11.html (accessed on 22 November 2023).
- Baskin, S.I.; Brewer, T.G. Cyanide Poisoning (From Medical Aspects of Chemical and Biological Warfare; Frederick, R., Sidell, M.D., Ernest, T., Takafuji, M.D., Eds.; NCJ: Amsterdam, The Netherlands, 1997; pp. 271–286. [Google Scholar]
- Sciuto, A.M.; Wong, B.J.; Martens, M.E.; Hoard-Fruchey, H.; Perkins, M.W. Phosphine toxicity: A story of disrupted mitochondrial metabolism. Ann. New York Acad. Sci. 2016, 1374, 41–51. [Google Scholar] [CrossRef]
- Kashi, K.; Chefurka, W. The effect of phosphine on the absorption and circular dichroic spectra of cytochrome c and cytochrome oxidase. Pestic. Biochem. Physiol. 1976, 6, 350–362. [Google Scholar] [CrossRef]
- Winks, R.; Waterford, C. The relationship between concentration and time in the toxicity of phosphine to adults of a resistant strain of Tribolium castaneum (Herbst). J. Stored Prod. Res. 1986, 22, 85–92. [Google Scholar] [CrossRef]
- Walse, S.S.; Jimenez, L.R. Postharvest fumigation of fresh citrus with cylinderized phosphine to control bean thrips (Thysanoptera: Thripidae). Horticulturae 2021, 7, 134. [Google Scholar] [CrossRef]
- Lampiri, E.; Agrafioti, P.; Athanassiou, C.G. Delayed mortality, resistance and the sweet spot, as the good, the bad and the ugly in phosphine use. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Bond, E.J.; Dumas, T.; Hobbs, S. Corrosion of metals by the fumigant phosphine. J. Stored Prod. Res. 1984, 20, 57–63. [Google Scholar] [CrossRef]
- Soderstrom, E.L.; Brandl, D.G.; Hartsell, P.L.; Mackey, B. Fumigants as treatments for harvested citrus fruits infested with Asynonychus godmani (Coleoptera: Curculionidae). J. Econ. Entomol. 1991, 84, 936–941. [Google Scholar] [CrossRef]
- Bell, C. The tolerance of developmental stages of four stored product moths to phosphine. J. Stored Prod. Res. 1976, 12, 77–86. [Google Scholar] [CrossRef]
- Su, N.-Y.; Scheffrahn, R.H. Efficacy of sulfuryl fluoride against four beetle pests of museums (Coleoptera: Dermestidae, Anobiidae). J. Econ. Entomol. 1990, 83, 879–882. [Google Scholar] [CrossRef]
- Bell, C.; Savvidou, N. The toxicity of Vikane (sulfuryl fluoride) to age groups of eggs of the Mediterranean flour moth (Ephestia kuehniella). J. Stored Prod. Res. 1999, 35, 233–247. [Google Scholar] [CrossRef]
- Baltaci, D.; Klementz, D.; Gerowitt, B.; Drinkall, M.; Reichmuth, C. Lethal effects of sulfuryl fluoride on eggs of different ages and other life stages of the warehouse moth Ephestia elutella (Hübner). J. Stored Prod. Res. 2009, 45, 19–23. [Google Scholar] [CrossRef]
- Bonjour, E.; Opit, G.; Hardin, J.; Jones, C.; Payton, M.; Beeby, R. Efficacy of ozone fumigation against the major grain pests in stored wheat. J. Econ. Entomol. 2011, 104, 308–316. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Phillips, T.W.; Aikins, M.J.; Hasan, M.M.; Throne, J.E. Effectiveness of sulfuryl fluoride for control of different life stages of stored-product psocids (Psocoptera). J. Econ. Entomol. 2012, 105, 282–287. [Google Scholar] [CrossRef]
- Kenaga, E.E. Time, temperature and dosage relationships of several insecticidal fumigants. J. Econ. Entomol. 1961, 54, 537–542. [Google Scholar] [CrossRef]
- Bell, C. Factors affecting the efficacy of sulphuryl fluoride as a fumigant. In Proceedings of the Ninth International Working Conference on Stored Product Protection, Sao Paulo, Brazil, 15–18 October 2006; pp. 519–526. [Google Scholar]
- Outram, I. Factors affecting the resistance of insect eggs to sulphuryl fluoride—I: The uptake of sulphuryl-35S fluoride by insect eggs. J. Stored Prod. Res. 1967, 3, 255–260. [Google Scholar] [CrossRef]
- Outram, I. Factors affecting the resistance of insect eggs to sulphuryl fluoride—II: The distribution of sulphuryl-35S fluoride in insect eggs after fumigation. J. Stored Prod. Res. 1967, 3, 353–358. [Google Scholar] [CrossRef]
- Gautam, S.; Opit, G.; Margosan, D.; Tebbets, J.; Walse, S. Egg morphology of key stored-product insect pests of the United States. Ann. Entomol. Soc. Am. 2014, 107, 1–10. [Google Scholar] [CrossRef]
- Gautam, S.; Opit, G.; Margosan, D.; Hoffmann, D.; Tebbets, J.; Walse, S. Comparative egg morphology and chorionic ultrastructure of key stored-product insect pests. Ann. Entomol. Soc. Am. 2015, 108, 43–56. [Google Scholar] [CrossRef]
- Hinton, H.E. Respiratory systems. Biol. Insect Eggs 1981, 1, 95–148. [Google Scholar]
- Trougakos, I.P.; Margaritis, L.H. Novel morphological and physiological aspects of insect eggs. In Chemoecology of Insect Eggs and Egg Deposition; Blackwell Publishing: Berlin, Germany, 2003; pp. 2–36. [Google Scholar]
- Kučerová, Z.; Stejskal, V. Comparative egg morphology of silvanid and laemophloeid beetles (Coleoptera) occurring in stored products. J. Stored Prod. Res. 2002, 38, 219–227. [Google Scholar] [CrossRef]
- Bozzola, J.J.; Russell, L.D. Electron Microscopy: Principles and Techniques for Biologists; Jones & Bartlett Learning: Burlington, MA, USA, 1999. [Google Scholar]
- Hayat, M.A. Principles and Techniques of Electron Microscopy: Biological Applications, 4th ed.; Cambridge University Press: Cambridge, UK, 2000; 543p. [Google Scholar]
- Matesco, V.C.; Fürstenau, B.; Bernardes, J.L.; Schwertner, C.F.; Grazia, J. Morphological features of the eggs of Pentatomidae (Hemiptera: Heteroptera). Zootaxa 2009, 1984, 1–30. [Google Scholar] [CrossRef]
- Matesco, V.C.; Bianchi, F.M.; Fürstenau, B.B.R.J.; Da Silva, P.P.; Campos, L.A.; Grazia, J. External egg structure of the Pentatomidae (Hemiptera: Heteroptera) and the search for characters with phylogenetic importance. Zootaxa 2014, 3768, 351–385. [Google Scholar] [CrossRef]
- Javahery, M. Development of eggs in some true bugs (Hemiptera–Heteroptera). Part I. Pentatomoidea. Can. Entomol. 1994, 126, 401–433. [Google Scholar] [CrossRef]
- Vilimova, J.; Rohanova, M. The external morphology of eggs of three Rhopalidae species (Hemiptera: Heteroptera) with a review of the eggs of this family. Acta Entomol. Musei Natl. Pragae 2010, 50, 75–95. [Google Scholar]
- Arbogast, R.T.; Lecato, G.L.; Van Byrd, R. External morphology of some eggs of stored-product moths (Lepidoptera Pyralidae, Gelechiidae, Tineidae). Int. J. Insect Morphol. Embryol. 1980, 9, 165–177. [Google Scholar] [CrossRef]








| Fumigant | Molecule Diameter (nm) | Molecular Area (nm2) | # Molecules per Pore (Billions) |
|---|---|---|---|
| ethyl formate | 0.557 | 0.2435 | 1.15 |
| sulfuryl fluoride | 0.259 | 0.0527 | 5.31 |
| phosphine | 0.255 | 0.0510 | 5.48 |
| methyl bromide | 0.221 | 0.0383 | 7.29 |
| hydrogen cyanide | 0.219 | 0.0376 | 7.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powell, J.M.; Nixon, L.J.; Lourie, A.P.; Leskey, T.C.; Walse, S.S. Egg Morphology and Chorionic Ultrastructure of Spotted Lanternfly, Lycorma delicatula (White) (Hemiptera: Fulgoridae). Forests 2023, 14, 2354. https://doi.org/10.3390/f14122354
Powell JM, Nixon LJ, Lourie AP, Leskey TC, Walse SS. Egg Morphology and Chorionic Ultrastructure of Spotted Lanternfly, Lycorma delicatula (White) (Hemiptera: Fulgoridae). Forests. 2023; 14(12):2354. https://doi.org/10.3390/f14122354
Chicago/Turabian StylePowell, Jonathan M., Laura J. Nixon, Austin P. Lourie, Tracy C. Leskey, and Spencer S. Walse. 2023. "Egg Morphology and Chorionic Ultrastructure of Spotted Lanternfly, Lycorma delicatula (White) (Hemiptera: Fulgoridae)" Forests 14, no. 12: 2354. https://doi.org/10.3390/f14122354
APA StylePowell, J. M., Nixon, L. J., Lourie, A. P., Leskey, T. C., & Walse, S. S. (2023). Egg Morphology and Chorionic Ultrastructure of Spotted Lanternfly, Lycorma delicatula (White) (Hemiptera: Fulgoridae). Forests, 14(12), 2354. https://doi.org/10.3390/f14122354