Daily Climatic Data Better Explain the Radial Growth of Swiss Stone Pine (Pinus cembra L.) in High-Elevation Cliffs in the Carpathians
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Climate
2.2. Tree-Ring and Climatic Data
2.3. Analysis of Daily Climate–Growth Relationships
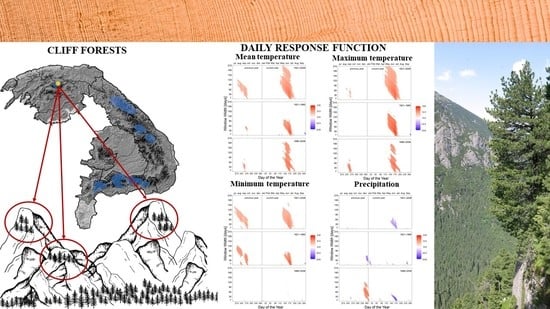
3. Results
3.1. Site-Specific Chronology
3.2. Climate–Growth Relationships
3.2.1. Mean Temperature
3.2.2. Minimum Temperature
3.2.3. Maximum Temperature
3.2.4. Precipitation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adler, C.; Wester, P.; Bhatt, I.D.; Huggel, C.; Insarov, G.; Morecroft, M.; Muccione, V.; Prakash, A.; Alcántara-Ayala, I.; Allen, S.K.; et al. Cross-Chapter Paper 5: Mountains. In Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, NY, USA, 2022; pp. 2273–2318. [Google Scholar] [CrossRef]
- Esper, J.; Klippel, L.; Krusic, P.J.; Konter, O.; Raible, C.C.; Xoplaki, E.; Luterbacher, J.; Büntgen, U. Eastern Mediterranean summer temperatures since 730 CE from Mt. Smolikas tree-ring densities. Clim. Dyn. 2020, 54, 1367–1382. [Google Scholar] [CrossRef]
- Chen, D.; Rojas, M.; Samset, B.H.; Cobb, K.; Diongue Niang, A.; Edwards, P.; Emori, S.; Faria, S.H.; Hawkins, E.; Hope, P.; et al. Framing, Context, and Methods. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, NY, USA, 2021; pp. 147–286. [Google Scholar] [CrossRef]
- Beniston, M.; Keller, F.; Koffi, B.; Goyette, S. Estimates of snow accumulation and volume in the Swiss Alps under changing climatic conditions. Theor. Appl. Climatol. 2003, 76, 125–140. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.; Esper, J. Characterization and climate response patterns of a high-elevation, multi-species tree-ring network in the European Alps. Dendrochronologia 2005, 22, 107–121. [Google Scholar] [CrossRef]
- Zscheischler, J.; Westra, S.; van den Hurk, B.J.; Seneviratne, S.I.; Ward, P.J.; Pitman, A.; AghaKouchak, A.; Bresch, D.N.; Leonard, M.; Wahl, T.; et al. Future climate risk from compound events. Nat. Clim. Chang. 2018, 8, 469–477. [Google Scholar] [CrossRef]
- Babst, F.; Bouriaud, O.; Poulter, B.; Trouet, V.; Girardin, M.P.; Frank, D.C. Twentieth century redistribution in climatic drivers of global tree growth. Sci. Adv. 2019, 5, eaat4313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camarero, J.J.; Linares, J.C.; Sangüesa-Barreda, G.; Sánchez-Salguero, R.; Gazol, A.; Navarro-Cerrillo, R.M.; Carreira, J.A. The multiple causes of forest decline in Spain: Drought, historical logging, competition and biotic stressors. In Dendroecology; Amoroso, M., Daniels, L., Baker, P., Camarero, J.J., Eds.; Springer: New York, NY, USA, 2017; pp. 307–323. [Google Scholar] [CrossRef]
- Casalegno, S.; Amatulli, G.; Camia, A.; Nelson, A.; Pekkarinen, A. Vulnerability of Pinus cembra L. in the Alps and the Carpathian Mountains under present and future climates. For. Ecol. Manag. 2010, 259, 750–761. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Marchetti, M. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H. (Ted); et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Spinoni, J.; Vogt, J.V.; Naumann, G.; Barbosa, P.; Dosio, A. Will drought events become more frequent and severe in Europe? Int. J. Climatol. 2018, 38, 1718–1736. [Google Scholar] [CrossRef] [Green Version]
- Schweingruber, F.H. Tree Rings and Environment: Dendroecology; Haupt-Verlag: Bern, Switzerland, 1996; p. 609. [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010; p. 368. [Google Scholar]
- Pearl, J.; Keck, J.; Tintor, W.; Siekacz, L.; Herrick, H.; Meko, D.; Pearson, C. New frontiers in tree-ring research. Holocene 2020, 30, 923–941. [Google Scholar] [CrossRef]
- Körner, C. Alpine Treelines: Functional Ecology of the Global High Elevation Tree Limits; Springer: Berlin/Heidelberg, Germany, 2012; p. 220. [Google Scholar]
- Tranquillini, W. Physiological ecology of the alpine timberline. In Ecological Study; Springer Nature: Berlin/Heidelberg, Germany; New York, NY, USA, 1979; Volume 31, pp. 1–137. [Google Scholar]
- Körner, C.; Paulsen, J.A. World-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Izworska, K.; Muter, E.; Matulewski, P.; Zielonka, T. Tree rings as an ecological indicator of the reaction of Swiss stone pine (Pinus cembra L.) to climate change and disturbance regime in the extreme environment of cliff forests. Ecol. Indic. 2023, 148, 110102. [Google Scholar] [CrossRef]
- Tóth, E.G.; Tremblay, F.; Housset, J.M.; Bergeron, Y.; Carcaillet, C. Geographic isolation and climatic variability contribute to genetic differentiation in fragmented populations of the long-lived subalpine conifer Pinus cembra L. in the western Alps. BMC Evol. Biol. 2019, 19, 190. [Google Scholar] [CrossRef] [Green Version]
- Zięba, A.; Różański, W.; Bukowski, M.; Pałka, B.; Szwagrzyk, J. Distribution and habitat conditions of Pinus cembra forests in the Tatra Mountains. Dendrobiology 2019, 81, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Carrer, M.; Unterholzner, L.; Castagneri, D. Wood anatomical traits highlight complex temperature influence on Pinus cembra at high elevation in the Eastern Alps. Int. J. Biometeorol. 2018, 62, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Carrer, M.; Urbinati, C. Age-dependent tree-ring growth responses to climate in Larix decidua and Pinus cembra. Ecology 2004, 85, 730–740. [Google Scholar] [CrossRef]
- Oberhuber, W.; Kofler, W.; Pfeifer, K.; Seeber, A.; Gruber, A.; Wieser, G. Long-term changes in tree-ring–climate relationships at Mt. Patscherkofel (Tyrol, Austria) since the mid-1980s. Trees 2008, 22, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Saulnier, M.; Edouard, J.L.; Corona, C.; Guibal, F. Climate/growth relationships in a Pinus cembra high-elevation network in the Southern French Alps. Ann. For. Sci. 2011, 68, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Popa, I.; Nechita, C.; Hofgaard, A. Stand structure, recruitment and growth dynamics in mixed subalpine spruce and Swiss stone pine forests in the Eastern Carpathians. Sci. Total Environ. 2017, 598, 1050–1057. [Google Scholar] [CrossRef]
- Popa, I.; Kern, Z. Long-term summer temperature reconstruction inferred from tree-ring records from the Eastern Carpathians. Clim. Dyn. 2009, 32, 1107–1117. [Google Scholar] [CrossRef]
- Roibu, C.C.; Popa, I.; Kirchhefer, A.J.; Palaghianu, C. Growth responses to climate in a tree-ring network of European beech (Fagus sylvatica L.) from the eastern limit of its natural distribution area. Dendrochronologia 2017, 42, 104–116. [Google Scholar] [CrossRef]
- Rozas, V. Dendrochronology of pedunculate oak (Quercus robur L.) in an old-growth pollarded woodland in northern Spain: Tree-ring growth responses to climate. Ann. For. Sci. 2005, 62, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Jevšenak, J.; Levanič, T. dendroTools: R package for studying linear and nonlinear responses between tree-rings and daily environmental data. Dendrochronologia 2018, 48, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Jevšenak, J. Daily climate data reveal stronger climate-growth relationships for an extended European tree-ring network. Quat. Sci. Rev. 2019, 221, 105868. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A.V. Growth dynamics of conifer tree rings: Images of past and future environments. In Ecological Study; Springer Nature: Berlin/Heidelberg, Germany; New York, NY, USA, 2006; Volume 183, p. 350. [Google Scholar]
- Niedzwiedz, T. Climate of the Tatra Mountains. Mt. Res. Dev. 1992, 12, 131–146. [Google Scholar] [CrossRef]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree-Ring Res. 2001, 57, 5–21. Available online: http://hdl.handle.net/10150/251654 (accessed on 21 April 2023).
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–75. [Google Scholar]
- Cornes, R.C.; van der Schrier, G.; van den Besselaar, E.J.; Jones, P.D. An ensemble version of the E-OBS temperature and precipitation data sets. J. Geophys. Res. Atmos. 2018, 123, 9391–9409. [Google Scholar] [CrossRef] [Green Version]
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org (accessed on 21 April 2023).
- McCarroll, D.; Loader, N.J. Stable isotopes in tree rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976; p. 582. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Buras, A. A comment on the expressed population signal. Dendrochronologia 2017, 44, 130–132. [Google Scholar] [CrossRef]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Bednarz, Z. Relationship of Tree-Ring Widths in the Tatra Mountains to Variations in Monthly Temperature and Precipitation; Mitteilungen der Forstlichen Bundes-Versuchsanstalt: Wien, Austria, 1981; Volume 142, pp. 367–389. [Google Scholar]
- Büntgen, U.; Trnka, M.; Krusic, P.J.; Kyncl, T.; Kyncl, J.; Luterbacher, J.; Zorita, E.; Ljungqvist, F.C.; Auer, I.; Konter, O.; et al. Tree-ring amplification of the early nineteenth-century summer cooling in Central Europe. J. Clim. 2015, 28, 5272–5288. [Google Scholar] [CrossRef] [Green Version]
- Știrbu, M.I.; Roibu, C.C.; Carrer, M.; Mursa, A.; Unterholzner, L.; Prendin, A.L. Contrasting climate sensitivity of Pinus cembra tree-ring traits in the Carpathians. Front. Plant Sci. 2022, 13, 855003. [Google Scholar] [CrossRef]
- Körner, C.; Hoch, G. Not every high-latitude or high-elevation forest edge is a treeline. J. Biogeogr. 2023, 50, 838–845. [Google Scholar] [CrossRef]
- Carrer, M.; Nola, P.; Eduard, J.L.; Motta, R.; Urbinati, C. Regional variability of climate–growth relationships in Pinus cembra high elevation forests in the Alps. J. Ecol. 2007, 95, 1072–1083. [Google Scholar] [CrossRef]
- Housset, J.M.; Tóth, E.G.; Girardin, M.P.; Tremblay, F.; Motta, R.; Bergeron, Y.; Carcaillet, C. Tree-rings, genetics and the environment: Complex interactions at the rear edge of species distribution range. Dendrochronologia 2021, 69, 125863. [Google Scholar] [CrossRef]
- Vittoz, P.; Rulence, B.; Largey, T.; Freléchoux, F. Effects of climate and land-use change on the establishment and growth of cembran pine (Pinus cembra L.) over the altitudinal treeline ecotone in the Central Swiss Alps. Arct. Antarct. Alp. Res. 2008, 40, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Mayr, S.; Hacke, U.; Schmid, P.; Schwienbacher, F.; Gruber, A. Frost drought in conifers at the alpine timberline: Xylem dysfunction and adaptations. Ecology 2006, 87, 3175–3185. [Google Scholar] [CrossRef] [Green Version]
- Oberhuber, W. Influence of climate on radial growth of Pinus cembra within the alpine timberline ecotone. Tree Physiol. 2004, 24, 291–301. [Google Scholar] [CrossRef]
- Simard, S.; Giovannelli, A.; Treydte, K.; Traversi, M.L.; King, G.M.; Frank, D.; Fonti, P. Intra-annual dynamics of non-structural carbohydrates in the cambium of mature conifer trees reflects radial growth demands. Tree Physiol. 2013, 33, 913–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Körner, C.; Hoch, G. A test of treeline theory on a montane permafrost island. Arct. Antarct. Alp. Res. 2006, 38, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Briffa, K.R.; Schweingruber, F.H.; Jones, P.D.; Osborne, T.J.; Shiyatov, S.G.; Vaganov, E.A. Reduced sensitivity of recent tree-growth to temperature at high northern latitudes. Nature 1998, 391, 678–682. [Google Scholar] [CrossRef]
- Paulsen, J.; Weber, U.M.; Körner, C. Tree growth near treeline: Abrupt or gradual reduction with altitude? Arct. Antarct. Alp. Res. 2000, 32, 14–20. [Google Scholar] [CrossRef]
- Körner, C. The cold range limit of trees. Trends Ecol. Evol. 2021, 36, 979–989. [Google Scholar] [CrossRef]
- Dzialuk, A.; Chybicki, I.; Gout, R.; Mączka, T.; Fleischer, P.; Konrad, H.; Curtu, L.; Sofletea, N.; Valadon, A. No reduction in genetic diversity of Swiss stone pine (Pinus cembra L.) in Tatra Mountains despite high fragmentation and small population size. Conserv. Genet. 2014, 15, 1433–1445. [Google Scholar] [CrossRef] [Green Version]
- Gugerli, F.; Brodbeck, S.; Lendvay, B.; Dauphin, B.; Bagnoli, F.; van der Knaap, W.O.; Tinner, W.; Höhn, M.; Vendramin, G.G.; Morales-Molino, C.; et al. A range-wide postglacial history of Swiss stone pine based on molecular markers and palaeoecological evidence. J. Biogeogr. 2023, 50, 1049–1062. [Google Scholar] [CrossRef]
- Zwijacz-Kozica, T.; Żywiec, M. Fifty-year changes in a strictly protected stone pine population in the Tatra National Park. Nat. Conserv. 2007, 64, 73–82. [Google Scholar]








| Parameter | Number of Years | Number of Trees | Interseries Correlation (Rbar) | Expressed Population Signal (EPS) | Subsample Signal Strength (SSS) | Mean Sensitivity (MS) |
|---|---|---|---|---|---|---|
| 1921–2009 | 89 | 104 | 0.16 | 0.951 | 0.998 | 0.196 |
| 1921–1965 | 45 | 104 | 0.17 | 0.953 | 0.997 | 0.187 |
| 1966–2009 | 44 | 104 | 0.14 | 0.942 | 0.998 | 0.188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izworska, K.; Zielonka, T.; Matulewski, P.; Muter, E. Daily Climatic Data Better Explain the Radial Growth of Swiss Stone Pine (Pinus cembra L.) in High-Elevation Cliffs in the Carpathians. Forests 2023, 14, 1411. https://doi.org/10.3390/f14071411
Izworska K, Zielonka T, Matulewski P, Muter E. Daily Climatic Data Better Explain the Radial Growth of Swiss Stone Pine (Pinus cembra L.) in High-Elevation Cliffs in the Carpathians. Forests. 2023; 14(7):1411. https://doi.org/10.3390/f14071411
Chicago/Turabian StyleIzworska, Katarzyna, Tomasz Zielonka, Paweł Matulewski, and Elżbieta Muter. 2023. "Daily Climatic Data Better Explain the Radial Growth of Swiss Stone Pine (Pinus cembra L.) in High-Elevation Cliffs in the Carpathians" Forests 14, no. 7: 1411. https://doi.org/10.3390/f14071411
APA StyleIzworska, K., Zielonka, T., Matulewski, P., & Muter, E. (2023). Daily Climatic Data Better Explain the Radial Growth of Swiss Stone Pine (Pinus cembra L.) in High-Elevation Cliffs in the Carpathians. Forests, 14(7), 1411. https://doi.org/10.3390/f14071411