Flavanols and Flavonols in the Nuclei of Conifer Genotypes with Different Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Histochemistry and Light Microscopy
2.3. HPLC Analysis of Flavonoids in Needles from Tsuga canadensis
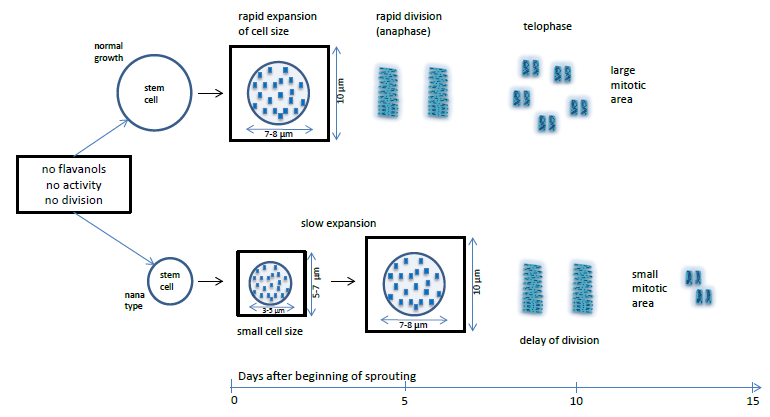
3. Results
3.1. Approximate Growth Parameters of the Species


3.2. Seasonal Changes of Phenolic Compounds from Entire Needles of Tsuga Canadensis and the Importance of Cell Structures

3.3. Micrometric Histology of Nuclear Flavanols from Needles of the Tsuga Genotypes

3.4. Micrometric Histology of Nuclear Flavanols from Needles of the Taxus Genotypes
4. Discussion
4.1. Nuclear Flavanols during the Metabolically Greatly Intensified S Phase
4.2. The Interplay of Cytokinin, Auxin, Sucrose, and Flavanols
4.3. Growth-Modifying Balance of Flavanols and Flavonols in Nuclei
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Krauze-Baranowska, M. Flavonoids from the genus Taxus. Z. Naturforsch C 2004, 59, 43–47. [Google Scholar]
- Hergert, H.L. Hemlock and spruce tannins: An odyssey. In Chemistry and Significance of Condensed Tannins; Hemmingway, R.W., Karchesy, J.J., Branham, S.J., Eds.; Plenum Press: New York, NY, USA, 1998; pp. 3–20. [Google Scholar]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; Feucht, W.; Polster, J.; Trnkova, L.; Burgos, P.; Parker, A.W.; Botchway, S.W. Two photon excitation with pico-second fluorescence lifetime imaging to detect nuclear association of flavanols. Anal. Chim. Acta 2012, 719, 68–75. [Google Scholar]
- Feucht, W.; Treutter, D.; Polster, J. Flavanols in nuclei of tree species: Facts and possible functions. Trees 2012, 26, 1413–1425. [Google Scholar] [CrossRef]
- Iglesias, M.J.; Terrille, M.C.; Bartoli, C.G.; D’Ippolito, S.; Casalongue, C.A. Auxin signaling participates in the adaptive response against oxidative stress and salinity with redox metabolism in Arabidopsis. Plant Mol. Biol. 2010, 74, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bidel, L.P.R.; Coumans, M.; Baissac, Y.; Doumas, P.; Jay-Allmand, C. Biological activity of phenolics in plant cells. In Recent Advances in Polyphenol Research; Santos-Buelga, C., Escribano-Bailon, M.T., Lattancio, V., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 3–205. [Google Scholar]
- Fini, A.; Brunetti, C.; Di Fernando, M.; Ferrini, F.; Tattini, M. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Feucht, W.; Treutter, D.; Dithmar, H.; Polster, J. Microspore development of three coniferous species: Affinity of nuclei for flavonoids. Tree Physiol. 2008, 28, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Feucht, W.; Treutter, D.; Dithmar, H.; Polster, J. Loss of nuclear flavanols during drought periods in Taxus baccata. Plant Biol. 2012, 14, 1–9. [Google Scholar] [PubMed]
- Lau, L.N.; Kan, C.W.; Juen, M.C.W.; Lau, K.W.; Lam, I.H.L.; Murreis, C.M. Study of the correlation between solid colors measured by Spectrometer and DigiEye. In Proceedings of the first International Conference on Interdisciplinary Research and Development, Bangkok, Thailand, 31 May–1 June 2011; pp. 31–36.
- Richard, T.; Vitrac, X.; Merrilon, J.M.; Monti, J.P. Role of peptide primary sequence in polyphenol-protein recognition: An example with neurotensin. Biochim. Biophys. Acta 2005, 1726, 238–243. [Google Scholar] [CrossRef]
- Polster, J.; Dithmar, H.; Feucht, W. Are histones the targets for flavan-3-ols (catechins) in nuclei? Biol. Chem. 2003, 384, 997–1006. [Google Scholar]
- Bohin, M.C.; Vinken, J.P.; van der Hidden, H.T.; Gruppen, H. Efficacy of food proteins as carriers for flavonoids. J. Agric. Food Chem. 2012, 60, 4136–4143. [Google Scholar] [CrossRef] [PubMed]
- Sussex, I.M.; Kerk, N.M. The organization and function of plant meristems. In Meristematic Tissues in Plant Growth and Development 2002; McManus, M.T., Veit, B.E., Eds.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Noctor, G.; De Paepe, R.; Foyer, C.H. Mitochrondrial redox biology and homeostasis in plants. Trends Plant Sci. 2007, 12, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Tyrakowska, B.; Muzolf, M.; Szymusiak, H. pH-Dependent Radical Scavenging Capacity of Epicatechin and Epicatechin Gallate. In Proceedings of the Conference on Polyphenol Communication Winnipeg, Manitoba, Canada, 22–25 August 2006.
- Feucht, W.; Treutter, D. Catechin effects on growth related processes in cultivated callus of Prunus avium. Gartenbauwiss 1995, 60, 7–11. [Google Scholar]
- Le Goff, L.; Roussaux, J.; Aaron-da Cunha, M.I.; Beljanski, M. Growth inhibition of crown-gall tissues in relation to the structure and activity of DNA. Physiol. Plant 1985, 64, 177–184. [Google Scholar]
- Leshem, Y.Y.; Halevy, A.H.; Frenkel, C. Progress and Control of Plant Senescence; Elsevier Publishing Company: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Skylar, A.; Hong, F.; Chory, J.; Weigel, D.; Wu, X. STIMPY mediates cytokinin signalling during shoot meristem establishment in Arabidopsis seedlings. Development 2010, 137, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Van Staden, J. Seeds and cytokinin. Physiol. Plant 1983, 58, 340–346. [Google Scholar]
- Lux-Endrich, A.; Treutter, D.; Feucht, W. Influence of nutrients and carbohydrate supply on the phenol composition of apple shoots. Plant Cell Tissue Organ Cult. 2000, 60, 15–21. [Google Scholar]
- Masuda, Y.; Kamisaka, S. Rapid stimulation of RNA biosynthesis by auxin. Plant Cell Physiol. 1969, 10, 79–86. [Google Scholar]
- Vanneste, S.; Friml, J. Auxin: A trigger for change in plant development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef]
- Tattini, M.; Gravano, E.; Pinelli, P.; Mulinacci, N.; Romani, A. Flavonoids accumulate in leaves and glandular trichomes of Phillyrea latifolia exposed to excess solar radiation. New Phytol. 2000, 148, 77–79. [Google Scholar] [CrossRef]
- Bi, S.; Qiao, C.; Song, D.; Tian, Y.; Gao, D.; Sun, Y.; Zhang, H. Study of interactions of flavonoids with DNA using acridine orange as a fluorescence probe. Sens. Act. B 2006, 119, 199–208. [Google Scholar] [CrossRef]
- Hur, W.; Gray, N.S. Small molecule modulators of antioxidant response pathways. Curr. Opin. Chem. Biol. 2011, 15, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ziegelin, G.; Schröder, W.; Frank, J.; Ayora, S.; Alonso, J.C.; Lanka, E.; Saenger, W. Flavones inhibit the hexameric replicative helicase RepA. Nucl. Acids Res. 2001, 29, 5058–5066. [Google Scholar] [CrossRef]
- Zapata-Torres, G.; Opazo, F.; Sagaldo, C.; Munoz, J.P.; Krautwurst, H. Effects of natural flavones and flavonols on the kinase activity of Cdk5. J. Nat. Prod. 2004, 67, 416–420. [Google Scholar]
- Shinozuka, K.; Kikuchi, Y.; Nishino, C.; Mori, A.; Tawata, S. Inhibitory effect of flavonoids on DNA-dependent DNA and RNA polymerases. Experientia 1988, 44, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.-E.; Wang, M.-X.; Li, R.-Q. Quercetin inhibits human SW 480 colon cancer growth in association with inhibition of cyclin D1 and surviving expression through Wnt/-Catenin signalling pathway. Cancer Investig. 2009, 27, 604–612. [Google Scholar] [CrossRef]
- Ackland, M.L.; van de Waarsenburg, S.; Jones, R. Synergistic antiproliferative action of the flavonols quercetin and kaempferol in cultured human cancer cell lines. Vivo 2006, 19, 69–76. [Google Scholar]
- Caltagirone, S.; Rossi, C.; Poggi, A.; Ranelletti, F.O.; Natali, P.G.; Brunetti, M.; Aiello, F.B.; Piantelli, M. Flavonoids apigenin and quercetin inhibit melanoma growth and metastasic potential. Int. J. Cancer 2000, 87, 595–600. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Feucht, W.; Schmid, M.; Treutter, D. Flavanols and Flavonols in the Nuclei of Conifer Genotypes with Different Growth. Forests 2014, 5, 2122-2135. https://doi.org/10.3390/f5092122
Feucht W, Schmid M, Treutter D. Flavanols and Flavonols in the Nuclei of Conifer Genotypes with Different Growth. Forests. 2014; 5(9):2122-2135. https://doi.org/10.3390/f5092122
Chicago/Turabian StyleFeucht, Walter, Markus Schmid, and Dieter Treutter. 2014. "Flavanols and Flavonols in the Nuclei of Conifer Genotypes with Different Growth" Forests 5, no. 9: 2122-2135. https://doi.org/10.3390/f5092122
APA StyleFeucht, W., Schmid, M., & Treutter, D. (2014). Flavanols and Flavonols in the Nuclei of Conifer Genotypes with Different Growth. Forests, 5(9), 2122-2135. https://doi.org/10.3390/f5092122