How Tightly Linked Are Pericopsis elata (Fabaceae) Patches to Anthropogenic Disturbances in Southeastern Cameroon?
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Species
2.2. Study Sites

2.3. Data Collection
2.4. Botanical Surveys
2.5. Charcoal Collection and Identification
2.6. Data Analysis
3. Results and Discussion
3.1. Floristic Inventory
| Characteristic | Species | Inside Patches | Outside Patches | ||||
|---|---|---|---|---|---|---|---|
| Site (surveyed area in ha) | Site 1 (2.12) | Site 2 (1.08) | Site 3 (1.26) | Site 1 (1.49) | Site 2 (0.99) | Site 3 (1.22) | |
| TD in n/ha | 287 | 342 | 405 | 395 | 416 | 423 | |
| MBA in m2 (SD in m2) | All species | 0.10 (0.01) | 0.08 (0.01) | 0.10 (0.01) | 0.06 (0.01) | 0.07 (0.01) | 0.07 (0.01) |
| P. elata individuals | 0.43 (0.17) | 0.39 (0.25) | 0.35 (0.27) | / | / | / | |
| MDBH in cm (SD in cm) | All species | 27.9 (1.7) | 25.7 (1.9) | 28.4 (1.8) | 22.8 (1.3) | 23.8 (1.7) | 24.7 (1.5) |
| P. elata individuals | 71.6 (18.0) | 66.2 (24.8) | 61.4 (27.2) | / | / | / | |
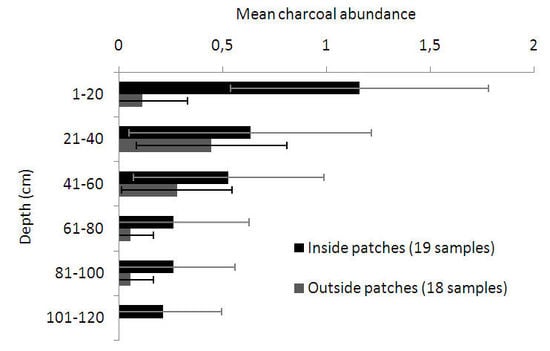
3.2. Anthracological Findings


| Site | Type of Sampling | Depth (cm) | Origin | Age 14C (BP) | Radiocarbon Laboratory Number |
|---|---|---|---|---|---|
| 1 | Auger | 41–60 | Seed | 1795 ± 40 (68.2%: 1632–1810 cal BP; 95.4%: 1609–1825 cal BP) * | KIA-38933 Z1S1 40–60 |
| 2 | Auger | 0–20 | Charcoal | 2150 ± 45 (68.2%: 2060–2301 cal BP; 95.4%: 2004–2308 cal BP) * | KIA-38938 L3S1 0–20 |
| 3 | Pit | 0–20 | Charcoal | 205 ± 30 (68.2%: 11–296 cal BP; 95.4%: 24–305 cal BP) * | KIA-38934 CAM. MIN 1.2 |
| 3 | Pit | 0–20 | Charcoal | 195 ± 30 (68.2%: 13–289 cal BP; 95.4%: 25–302 cal BP) * | KIA-38942 F4 200–220 |
3.3. Archaeological Findings
3.4. Autecology of P. elata

3.5. Anthracology and Archaeology Outcomes: The Likely Human-Induced Origin of P. elata Populations
3.6. Important Issues Regarding the Methodology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maley, J.; Brenac, P. Vegetation dynamics, palaeoenvironments and climatic changes in the forests of western Cameroon during the last 28,000 years BP. Rev. Palaeobot. Palynol. 1998, 99, 157–187. [Google Scholar]
- Nguetsop, V.F.; Servant-Vildary, S.; Servant, M. Late Holocene climatic changes in West Africa, a high resolution diatom record from equatorial Cameroon. Quat. Sci. Rev. 2004, 23, 591–609. [Google Scholar] [CrossRef]
- Clist, B. Traces de très anciennes occupations humaines de la forêt tropicale au Gabon. In Central African Hunter-Gatherers in a Multidisciplinary Perspective: Challenging Elusiveness; Biesbrouck, K., Elders, S., Rossel, G., Eds.; Research School for Asian, African and Amerindian Studies: Leiden, The Netherlands, 1999; pp. 75–87. [Google Scholar]
- Van Gemerden, B.S.; Olff, H.; Parren, M.P.E.; Bongers, F. The pristine rain forest? Remnants of historical human impacts on current tree species composition and diversity. J. Biogeogr. 2003, 30, 1381–1390. [Google Scholar]
- Brncic, T.M.; Willis, K.J.; Harris, D.J.; Washington, R. Culture or climate? The relative influences of past processes on the composition of the lowland Congo rainforest. Philos. Trans. R. Soc. Lond. Ser. B 2007, 36, 229–242. [Google Scholar] [CrossRef]
- Verdu, P.; Austerlitz, F.; Estoup, A.; Vitalis, R.; Georges, M.; Théry, S.; Froment, A.; Le Bomin, S.; Gessain, A.; Hombert, J.M.; et al. Origins and genetic diversity of Pygmy hunter-gatherers from Western Central Africa. Curr. Biol. 2009, 19, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Ngomanda, A.; Jolly, D.; Bentaleb, I.; Chepstow-Lusty, A.; Makaya, M.; Maley, J.; Fontugne, M.; Oslisly, R.; Rabenkogo, N. Lowland rainforest response to hydrological changes during the last 1500 years in Gabon, Western Equatorial Africa. Quat. Res. 2007, 67, 411–425. [Google Scholar] [CrossRef]
- Ngomanda, A.; Neumann, K.; Schweizer, A.; Maley, J. Seasonality change and the third millennium BP rainforest crisis in southern Cameroon (Central Africa). Quat. Res. 2009, 71, 307–318. [Google Scholar] [CrossRef]
- Neumann, K.; Bostoen, K.; Höhn, A.; Kahlheber, S.; Ngomanda, A.; Tchiengué, B. First farmers in the Central African rainforest: A view from southern Cameroon. Quat. Int. 2012, 249, 53–62. [Google Scholar] [CrossRef]
- De Wasseige, C.; de Marcken, P.; Bayol, N.; Hiol Hiol, F.; Mayaux, P.; Desclée, B.; Nasi, R.; Billand, A.; Defourny, P.; Eba’a, R. Les Forêts du Bassin du Congo—Etat des Forêts 2010; Office des Publications de l’Union Européenne: Luxembourg, Luxembourg, 2012. [Google Scholar]
- Réjou-Méchain, M.; Flores, O.; Bourland, N.; Doucet, J.-L.; Fétéké, R.; Pasquier, A.; Hardy, O.J. Spatial aggregation of tropical trees at multiple spatial scales. J. Ecol. 2011, 99, 1373–1381. [Google Scholar] [CrossRef]
- Fétéké, F.; Philippart, J. Plan D’aménagement des Unités Forestières D’aménagement n°10030 et 10031 Regroupées; Cellule d’aménagement Pallisco and Nature Plus: Mindourou, Cameroon and Gembloux, Belgium, 2008. [Google Scholar]
- Clist, B. Gabon: 100,000 ans d’Histoire; Centre Culturel français Saint Exupéry/Ministère de la Coopération/Sépia: Libreville, Gabon, 1995. [Google Scholar]
- Schwartz, D.; Lanfranchi, R. Les cadres paléoenvironnementaux de l’évolution humaine en Afrique Centrale Atlantique. LAnthropologie 1993, 97, 17–50. [Google Scholar]
- Horn, S.P.; Sanford, R.L. Holocene fires in Costa Rica. Biotropica 1992, 24, 354–361. [Google Scholar] [CrossRef]
- Tutin, C.E.; White, L.J.; Mackanga-Missandzou, A. Lightning strike burns large forest tree in the Lope Reserve, Gabon. Glob. Ecol. Biogeogr. Lett. 1996, 5, 36–41. [Google Scholar] [CrossRef]
- Cochrane, M.A.; Ryan, K.C. Tropical fire ecology. In Climate Change, Land Use, and Ecosystem Dynamics; Cochrane, M.A., Ed.; Praxis Publishing Ltd.: Chichester, UK, 2009; pp. 25–62. [Google Scholar]
- White, L.J.T.; Oates, J. New data on the history of the plateau forest of Okomu, southern Nigeria: An insight into how human disturbance has shaped the African rain forest. Glob. Geogr. Biogeogr. 1999, 8, 355–361. [Google Scholar] [CrossRef]
- Cerutti, P.O.; Nasi, R.; Tacconi, L. Sustainable forest management in Cameroon needs more than approved forest management plans. Ecol. Soc. 2008, 13, 36. [Google Scholar]
- Sist, P.; Ferreira, F.N. Sustainability of reduced-impact logging in the Eastern Amazon. For. Ecol. Manag. 2007, 243, 199–209. [Google Scholar] [CrossRef]
- Ouédraogo, D.-Y.; Fayolle, A.; Daïnou, K.; Demaret, C.; Bourland, N.; Lagoute, P.; Doucet, J.-L. Enrichment of logging gaps with a high conservation value species (Pericopsis elata) in a central African moist forest. Forests 2014, 5, 3031–3047. [Google Scholar] [CrossRef]
- Bourland, N.; Kouadio, Y.L.; Colinet, G.; Doucet, J.-L. Pericopsis elata (Harms) Meeuwen in the south-eastern part of Cameroon: Ecological and pedological approaches to improve the management of an endangered commercial timber species. Int. For. Rev. 2010, 12, 111. [Google Scholar]
- Hawthorne, W.D. Ecological Profiles of Ghanaian Forest Trees; Tropical Forestry Papers No. 29; Oxford Forestry Institute: Oxford, UK, 1995. [Google Scholar]
- Swaine, M.D. Rainfall and soil fertility as factors limiting forest species distributions in Ghana. J. Ecol. 1996, 84, 419–428. [Google Scholar] [CrossRef]
- Ampofo, S.T.; Lawson, G.W. Growth of seedlings of Afrormosia elata Harms in relation to light intensity. J. Appl. Ecol. 1972, 9, 301–306. [Google Scholar] [CrossRef]
- Kyereh, B.; Swaine, M.D.; Thompson, J. Effect of light on the germination of forest trees in Ghana. J. Ecol. 1999, 87, 772–783. [Google Scholar] [CrossRef]
- Bourland, N.; Kouadio, Y.L.; Lejeune, P.; Sonké, B.; Philippart, J.; Daïnou, K.; Fétéké, F.; Doucet, J.-L. Ecology of Pericopsis elata (Fabaceae), an endangered timber species in south-eastern Cameroon. Biotropica 2012, 44, 840–847. [Google Scholar] [CrossRef]
- Kouadio, Y.L.; Doucet, J.-L. Etude du comportement de Baillonella toxisperma Pierre (moabi) dans les trouées d’abattage enrichies. Biotechnol. Agron. Soc. Environ. 2009, 13, 317–324. [Google Scholar]
- White, F. The Vegetation of Africa; UNESCO: Paris, France, 1983. [Google Scholar]
- Grubb, P. Refuges and dispersal in the speciation of African forest mammals. In Biological Diversification in the Tropics; Prance, G.T., Ed.; Columbia University Press: New York, NY, USA, 1982; pp. 537–553. [Google Scholar]
- Chapman, C.A. Speciation of tropical rainforest primates of Africa: Insular biogeography. Afr. J. Ecol. 1983, 21, 297–308. [Google Scholar] [CrossRef]
- Forbes, M.S.; Raison, R.J.; Skjemstad, J.O. Formation, transformation and transport of black carbon (charcoal) in terrestrial and aquatic ecosystems. Sci. Total Environ. 2006, 370, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Brncic, T.M. Ecology and Patch Dynamics of Megaphrynium Macrostachyum (Benth.) Milne-Redh. (Marantaceae) in the South-West Central African Republic. Ph.D. Thesis, Oxford University, Oxford, UK, June 2002. [Google Scholar]
- Hubau, W.; Van den Bulcke, J.; Kitin, P.; Mees, F.; Van Acker, J.; Beeckman, H. Charcoal identification in species-rich biomes: A protocol for Central Africa optimised for the Mayumbe forest. Rev. Palaeobot. Palynol. 2012, 171, 164–178. [Google Scholar] [CrossRef]
- Chronometric Dating in Archaeology; Taylor, R.E.; Aitken, M. (Eds.) Advances in Archaeological and Museum Science; Springer-Verlag New York Inc.: New York, NY, USA, 1997; Volume 2.
- Bronk Ramsey, C.B. Bayesian analysis of radiocarbon dates. Radiocarbon 2009, 51, 337–360. [Google Scholar]
- Wolda, H. Similarity indices, sample size and diversity. Oecologia 1981, 50, 296–302. [Google Scholar] [CrossRef]
- BiodivR, Version 1.2; A program to compute statistically unbiased indices of species diversity within sample and species similarity between samples using rarefaction principles; Evolutionary Biology & Ecology: Brussels, Belgium, 2010. Available online: http://ebe.ulb.ac.be/ebe/Software.html (accessed on 13 October 2014).
- Cottam, G.; Curtis, J.T. The use of distance measures in phytosociological sampling. Ecology 1956, 37, 451–460. [Google Scholar] [CrossRef]
- Hall, J.B.; Bada, S.O. The distribution and ecology of Obeche (Triplochiton scleroxylon). J. Ecol. 1979, 67, 543–564. [Google Scholar] [CrossRef]
- Boyemba, F. Écologie de Pericopsis Elata (Harms) Van Meeuwen (Fabaceae), Arbre de Forêt Tropicale Africaine à Répartition Agrégée. Ph.D. Thesis, Université Libre de Bruxelles, Université de Kisangani, Brussels, Belgium, August 2011. [Google Scholar]
- Nyemeck Binam, J.; Tonye, J.; wandji, N.; Nyambi, G.; Akoa, M. Factors affecting the technical efficiency among smallholder farmers in the slash and burn agriculture zone of Cameroon. Food Policy 2004, 29, 531–545. [Google Scholar] [CrossRef]
- Clist, B. The Okala site, Estuaire province, Gabon. Its importance for understanding the transition to sedentarization in central Africa. Comptes Rendus l’Académie Sci. Ser. IIA Earth Planet. Sci. 1997, 325, 151–156. [Google Scholar]
- Oslisly, R.; White, L.J.T. La Relation Home/Milieu dans la Réserve de la Lopé (Gabon) au Cours de L’Holocène: Les Implications sur L’environnement. Dynamique à Long Terme des Écosystèmes Forestiers Intertropicaux; ORSTOM: Paris, France, 1996. [Google Scholar]
- Hart, T.B.; Hart, J.A.; Dechamps, R.; Fournier, M.; Ataholo, M. Changes in forest composition over the last 4000 years in the Ituri basin, Zaïre. In The Biodiversity of African Plants; Van der Maesen, L.J.G., Van der Burgt, X.M., Van Medenbach de Rooy, J.M., Eds.; Springer-Verlag: Heidelberg, Germany, 1996; pp. 545–563. [Google Scholar]
- Meister, C.; Eggert, M. On the early iron age in southern Cameroun: The site of Akonétye. J. Afr. Archaeol. 2008, 6, 183–202. [Google Scholar] [CrossRef]
- Oslisly, R.; Bentaleb, I.; Favier, C.; Fontugne, M.; Gillet, J.-F.; Morin-Rivat, J. West Central African peoples: Survey of radiocarbon dates over the past 5000 years. Radiocarbon 2013, 55, 1377–1382. [Google Scholar] [CrossRef]
- Newbery, D.M.; Van Der Burgt, X.M.; Worbes, M.; Chuyong, G.B. Transient dominance in a central African rain forest. Ecol. Monogr. 2013, 83, 339–382. [Google Scholar] [CrossRef]
- Livingstone Smith, A. Histoire du décor à la roulette en Afrique subsaharienne. J. Afr. Archaeol. 2007, 5, 189–216. [Google Scholar]
- CTFT. Fiche botanique, forestière, industrielle et commerciale: Assamela. Bois For. Trop. 1956, 50, 17–20. [Google Scholar]
- Dupuy, B. Bases Pour une Sylviculture en Forêt Dense Tropicale Humide Africaine; Série FORAFRI, Doc. 4; CIRAD-Forêt: Montpellier, France, 1998. [Google Scholar]
- Chao, A. Species richness estimation. In Encyclopedia of Statistical Sciences; Balakrishnan, N., Read, C.B., Vidakovic, B., Eds.; Wiley: Hoboken, NJ, USA, 2005; pp. 7909–7916. [Google Scholar]
- Vleminckx, J.; Morin-Rivat, J.; Biwolé, A.B.; Daïnou, K.; Gillet, J.-F.; Doucet, J.-L.; Drouet, T.; Hardy, O.J. Soil charcoal to assess the impacts of past human disturbances on tropical forests. PLoS One 2014, 9, e108121. [Google Scholar] [CrossRef] [PubMed]
- Balee, W. Cultural Forests of the Amazon: A Historical Ecology of People and Their Landscapes; University of Alabama Press: Tuscaloosa, AL, USA, 2013. [Google Scholar]
- Hunt, C.O.; Rabett, R.J. Holocene landscape intervention and plant food production strategies in island and mainland Southeast Asia. J. Archaeol. Sci. 2014, 51, 22–33. [Google Scholar] [CrossRef]
- McFadgen, B.G. Dating New Zealand archaeology by radiocarbon. N. Z. J. Sci. 1982, 25, 379–392. [Google Scholar]
- Gavin, D.G. Estimation of inbuilt age in radiocarbon ages of soil charcoal for fire history studies. Radiocarbon 2001, 43, 27–44. [Google Scholar]
- Tans, P.P.; de Jong, A.F.M.; Mook, W.G. Natural atmospheric 14C variation and the Suess effect. Nature 1979, 280, 826–828. [Google Scholar] [CrossRef]
- Fichtler, E.; Clark, D.A.; Worbes, M. Age and long-term growth of trees in an old-growth tropical rain forest, based on analyses of tree rings and 14C. Biotropica 2003, 35, 306–317. [Google Scholar] [CrossRef]
- Stuiver, M.; Becker, B. High-precision decadal calibration of the radiocarbon time scale, AD 1950–6000 BC. Radiocarbon 1993, 35, 35–65. [Google Scholar]
- Petchey, F.; Anderson, A.; Hogg, A.; Zondervan, A. The marine reservoir effect in the Southern Ocean: An evaluation of extant and new R values and their application to archaeological chronologies. J. R. Soc. N. Z. 2008, 38, 243–262. [Google Scholar] [CrossRef]
- De Ridder, M.; Toirambe, B.; van den Bulcke, J.; Bourland, N.; van Acker, J.; Beeckman, H. Dendrochronological potential in a semi-deciduous rainforest: The case of Pericopsis elata in central Africa. Forests 2014, 5, 3087–3106. [Google Scholar] [CrossRef] [Green Version]
- Gérard, P. Etude Écologique de la Forêt Dense à Gilbertiodendron Dewevrei dans la Région de l’Uele; Publications de l’Institut National pour l’Etude Agronomique du Congo: Yangambi, Belgian Congo, 1960; Volume 87, pp. 1–159. [Google Scholar]
- Hart, T.B.; Hart, J.A.; Murphy, P.G. Monodominant and species-rich forests of the humid tropics: Causes for their co-occurrence. Am. Nat. 1989, 133, 613–633. [Google Scholar] [CrossRef]
- Germain, R.; Evrard, C. Etude Écologique et Phytosociologique de la Forêt à Brachystegia Laurentii; Publications de l’Institut National pour l’Etude Agronomique du Congo Belge, INEAC: Yangambi, Belgian Congo, 1956. [Google Scholar]
- Biwolé, A.B.; Morin-Rivat, J.; Fayolle, A.; Bitondo, D.; Dedry, L.; Daïnou, K.; Hardy, O.J.; Doucet, J.-L. New data on the recent history of the littoral forests of southern Cameroon: An insight into the role of historical human disturbances on the current forest composition. Plant Ecol. Evol. 2015. [Google Scholar] [CrossRef]
- Karsenty, A.; Gourlet-Fleury, S. Assessing sustainability of logging practices in the Congo Basin’s managed forests: The issue of commercial species recovery. Ecol. Soc. 2006, 11, 26. [Google Scholar]
- Doucet, J.-L.; Ntchandi Otimbo, P.A.; Boubady, A.-G. Comment assister la régénération naturelle de l’okoumé dans les concessions forestières? Bois For. Trop. 2004, 279, 59–72. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourland, N.; Cerisier, F.; Daïnou, K.; Smith, A.L.; Hubau, W.; Beeckman, H.; Brostaux, Y.; Fayolle, A.; Biwolé, A.B.; Fétéké, F.; et al. How Tightly Linked Are Pericopsis elata (Fabaceae) Patches to Anthropogenic Disturbances in Southeastern Cameroon? Forests 2015, 6, 293-310. https://doi.org/10.3390/f6020293
Bourland N, Cerisier F, Daïnou K, Smith AL, Hubau W, Beeckman H, Brostaux Y, Fayolle A, Biwolé AB, Fétéké F, et al. How Tightly Linked Are Pericopsis elata (Fabaceae) Patches to Anthropogenic Disturbances in Southeastern Cameroon? Forests. 2015; 6(2):293-310. https://doi.org/10.3390/f6020293
Chicago/Turabian StyleBourland, Nils, François Cerisier, Kasso Daïnou, Alexandre Livingstone Smith, Wannes Hubau, Hans Beeckman, Yves Brostaux, Adeline Fayolle, Achille Bernard Biwolé, Fousséni Fétéké, and et al. 2015. "How Tightly Linked Are Pericopsis elata (Fabaceae) Patches to Anthropogenic Disturbances in Southeastern Cameroon?" Forests 6, no. 2: 293-310. https://doi.org/10.3390/f6020293
APA StyleBourland, N., Cerisier, F., Daïnou, K., Smith, A. L., Hubau, W., Beeckman, H., Brostaux, Y., Fayolle, A., Biwolé, A. B., Fétéké, F., Gillet, J. -F., Morin-Rivat, J., Lejeune, P., Tiba, E. N., Van Acker, J., & Doucet, J. -L. (2015). How Tightly Linked Are Pericopsis elata (Fabaceae) Patches to Anthropogenic Disturbances in Southeastern Cameroon? Forests, 6(2), 293-310. https://doi.org/10.3390/f6020293