How Do Urban Forests Compare? Tree Diversity in Urban and Periurban Forests of the Southeastern US
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Statistical Analyses
3. Results
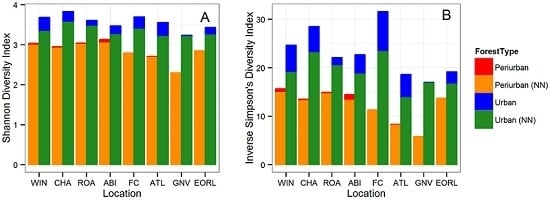
3.1. Tree Diversity Comparisons
3.2. Community Structure and Composition
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DBH | Diameter at breast height |
| FIA | Forest Inventory and Analysis |
| PERMANOVA | Permutational Analysis of Variance |
| PF | Periurban forests |
| SE US | Southeastern United States |
| UF | Urban forests |
| US | United States |
| USDA | United States Department of Agriculture |
References
- Wear, D.N. Forecasts of Land Uses. In The Southern Forest Futures Project: Technical Report; Greis, J.G., Ed.; USDA Forest Service: Washington, DC, USA, 2013; pp. 45–72. [Google Scholar]
- Conway, T.M.; Bourne, K.S. A comparison of neighborhood characteristics related to canopy cover, stem density and species richness in an urban forest. Landsc. Urban Plan. 2013, 113, 10–18. [Google Scholar] [CrossRef]
- Groffman, P.M.; Cavender-Bares, J.; Bettez, N.D.; Grove, J.M.; Hall, S.J.; Heffernan, J.B.; Hobbie, S.E.; Larson, K.L.; Morse, J.L.; Neill, C.; et al. Ecological homogenization of urban USA. Front. Ecol. Environ. 2014, 12, 74–81. [Google Scholar] [CrossRef]
- Richardson, D.M.; Rejmanek, M. Trees and shrubs as invasive alien species—A global review. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Raupp, M.; Cumming, A.; Raupp, E. Street Tree Diversity in Eastern North America and Its Potential for Tree Loss to Exotic Borers. Arboric. Urban For. 2006, 32, 297–304. [Google Scholar]
- Subburayalu, S.; Sydnor, T.D. Assessing street tree diversity in four Ohio communities using the weighted Simpson index. Landsc. Urban Plan. 2012, 106, 44–50. [Google Scholar] [CrossRef]
- Alvey, A.A. Promoting and preserving biodiversity in the urban forest. Urban For. Urban Green. 2006, 5, 195–201. [Google Scholar] [CrossRef]
- Thompson, I.; Mackey, B.; McNulty, S.; Mosseler, A. Forest Resilience, Biodiversity, and Climate Change. In A Synthesis of the Biodiversity/Resilience/Stability Relationship in Forest Ecosystems; Technical Series No. 43; Secretariat of the Convention on Biological Diversity: Montreal, Canada, 2009; p. 67. [Google Scholar]
- McPherson, E.; Simpson, J.R. Carbon dioxide reduction through urban forestry: Guidelines for professional and volunteer tree planters. Gen. Tech. Rep. 1999, 171, 237. [Google Scholar]
- Dreistadt, S.H.; Dahlsten, D.L.; Frankie, G.W. Urban Forests and Insect Ecology. Bioscience 2015, 40, 192–198. [Google Scholar] [CrossRef]
- Kendal, D.; Dobbs, C.; Lohr, V.I. Global patterns of diversity in the urban forest: Is there evidence to support the 10/20/30 rule? Urban For. Urban Green. 2014, 13, 411–417. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Cumming, G.S.; Olsson, P.; Chapin, F.S.; Holling, C.S. Resilience, experimentation, and scale mismatches in social-ecological landscapes. Landsc. Ecol. 2013, 28, 1139–1150. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Motard, E.; Muratet, A.; Clair-Maczulajtys, D.; MacHon, N. Does the invasive species Ailanthus altissima threaten floristic diversity of temperate peri-urban forests? Comptes Rendus-Biol. 2011, 334, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, M.; Larson, B.M.H.; Irlich, U.M.; Holmes, P.M.; Stafford, L.; van Wilgen, B.W.; Richardson, D.M. Managing invasive species in cities: A framework from Cape Town, South Africa. Landsc. Urban Plan. 2016, 151, 1–9. [Google Scholar] [CrossRef]
- Staudhammer, C.L.; Escobedo, F.J.; Holt, N.; Young, L.J.; Brandeis, T.J.; Zipperer, W. Predictors, spatial distribution, and occurrence of woody invasive plants in subtropical urban ecosystems. J. Environ. Manag. 2015, 155, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Vitousek, P.; D’Antonio, C.; Loope, L.L.; Westbrooks, R. Biological invasions as global environmental change. Am. Sci. 1996, 84, 468–478. [Google Scholar]
- Crossman, N.D.; Bryan, B.A.; Cooke, D.A. An invasive plant and climate change threat index for weed risk management: Integrating habitat distribution pattern and dispersal process. Ecol. Indic. 2011, 11, 183–198. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Kroeger, T.; Wagner, J.E. Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environ. Pollut. 2011, 159, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Horn, J.; Escobedo, F.J.; Hinkle, R.; Hostetler, M.; Timilsina, N. The Role of Composition, Invasives, and Maintenance Emissions on Urban Forest Carbon Stocks. Environ. Manag. 2015, 55, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Schierenbeck, K.A.; Ellstrand, N.C. Hybridization and the evolution of invasiveness in plants and other organisms. Biol. Invasions 2009, 11, 1093–1105. [Google Scholar] [CrossRef]
- Vincent, M.A. On the Spread and Current Distribution of Pyrus calleryana in the United States. Castanea 2005, 70, 20–31. [Google Scholar] [CrossRef]
- Thuiller, W. Biodiversity: Climate change and the ecologist. Nature 2007, 448, 550–552. [Google Scholar] [CrossRef] [PubMed]
- Konijnendijk, C.C.; Ricard, R.M.; Kenney, A.; Randrup, T.B. Defining urban forestry—A comparative perspective of North America and Europe. Urban For. Urban Green. 2006, 4, 93–103. [Google Scholar] [CrossRef]
- Ricketts, T.; Imhoff, M. Biodiversity, urban areas, and agriculture: Locating priority ecoregions for conservation. Ecol. Soc. 2003, 8, 1. [Google Scholar]
- Currie, D.J.; Paquin, V. Large-scale biogeographical patterns of species richness of trees. Nature 1987, 329, 326–327. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Nowak, D.; Crane, D.; Stevens, J.C.; Hoehn, R.E.; Walton, J.T.; Bond, J. A Ground-Based Method of Assessing Urban Forest Structure and Ecosystem Services. Arboric. Urban For. 2008, 34, 347–358. [Google Scholar]
- Baró, F.; Chaparro, L.; Gómez-Baggethun, E.; Langemeyer, J.; Nowak, D.J.; Terradas, J. Contribution of ecosystem services to air quality and climate change mitigation policies: The case of urban forests in Barcelona, Spain. Ambio 2014, 43, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.A.; Chappelka, A.H.; Keever, G.J.; Loewenstein, E.F. A 100 % Tree Inventory Using i-Tree Eco Protocol: A Case Study at Auburn University, Alabama, U.S. Arboric. Urban For. 2011, 37, 207–212. [Google Scholar]
- Lawrence, A.B.; Escobedo, F.J.; Staudhammer, C.L.; Zipperer, W. Analyzing growth and mortality in a subtropical urban forest ecosystem. Landsc. Urban Planing 2012, 104, 85–94. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Adams, D.C.; Timilsina, N. Urban forest structure effects on property value. Ecosyst. Serv. 2015, 12, 209–217. [Google Scholar] [CrossRef]
- Timilsina, N.; Staudhammer, C.L.; Escobedo, F.J.; Lawrence, A. Tree biomass, wood waste yield, and carbon storage changes in an urban forest. Landsc. Urban Planing 2014, 127, 18–27. [Google Scholar] [CrossRef]
- Wiseman, P.E.; King, J. i-Tree Ecosystem Analysis: Town of Abingdon, Urban Forest Effects and Values; Virginia Tech College of Natual Resources and Environment: Blacksburg, VA, USA, 2012. [Google Scholar]
- Urban Waters Partnership: Proctor Creek Watershed/Atlanta (Georgia). Available online: https://www.epa.gov/urbanwaterspartners/proctor-creek-watershedatlanta-georgia (accessed on 5 May 2016).
- Bailey, R.G. Identifying ecoregion boundaries. Environ. Manag. 2005, 34, 14–26. [Google Scholar] [CrossRef] [PubMed]
- US Census Bureau. TIGER/Line Shapefiles (machine-readable data files), 2012. Available online: https://www2.census.gov/geo/pdfs/maps-data/data/tiger/tgrshp2010/TGRSHP10SF1.pdf (accessed on 6 April 2016).
- Forest Inventory and Analysis Database. Available online: http://apps.fs.fed.us/fiadb-downloads/datamart.html (accessed on 6 April 2016).
- Delphin, S.; Escobedo, F.J.; Abd-Elrahman, A.; Cropper, W.P. Urbanization as a land use change driver of forest ecosystem services. Land Use Policy 2016, 54, 188–199. [Google Scholar] [CrossRef]
- McRoberts, R.; Holden, G.; Nelson, M.D.; Liknes, G.C.; Moser, W.K.; Lister, A.J.; King, S.L.; Lapoint, E.; Coulston, J.W.; Smith, W.B.; et al. Estimating and circumventing the effects of perturbing and swapping inventory plot locations. J. For. 2005, 103, 275–279. [Google Scholar]
- Woudenberg, S.W.; Conkling, B.L.; O’Connell, B.M.; LaPoint, E.B.; Turner, J.A.; Waddell, K.L.; Boyer, D.; Christensen, G.; Ridley, T. FIA database description and users manual for Phase 2. Version 5.1. US Department of Agriculture: Washington, DC, USA, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2013; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Vegan: Community Ecology Package, Version 2.3-5. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 3 March 2016).
- Invasive Plant Atlas of the United States. Available online: http://www.invasiveplantatlas.org/ (accessed on 7 June 2016).
- SAS/STAT®. SAS Institute Inc: Cary, NC, USA, 2011. Available online: http://www.sas.com/en_sg/software/analytics/stat.html (accessed on 7 June 2016).
- Nowak, D.; Crane, D. The Urban Forest Effects (UFORE) Model: Quantifying urban forest structure and functions. In Integrated Tools for Natural Resources Inventories in the 21st Century; Hansen, M., Burk, T., Eds.; US Department of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 2000; pp. 714–720. [Google Scholar]
- USDA, N. The PLANTS Database. Available online: http://plants.usda.gov (accessed on 20 March 2016).
- FLEPPC List of Invasive Plant Species 2015. Available online: www.fleppc.org (accessed on 7 June 2016).
- GEPPC List of Non-native Invasive Plants in Georgia. Available online: http://www.gainvasives.org/species/weeds/ (accessed on 1 February 2016).
- Hefferman, K.; Engle, E.; Richardson, C. Virginia Invasive Plant Species List; Virginia Department of Conservation and Recreation: Richmond, VA, USA, 2014.
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Chase, J.M.; Kraft, N.J.B.; Smith, K.G.; Vellend, M.; Inouye, B.D. Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere 2011, 2. [Google Scholar] [CrossRef]
- Raup, D.M.; Crick, R.E. Measurement of faunal similarity in paleontology. J. Paleontol. 1979, 53, 1213–1227. [Google Scholar]
- Vellend, M.; Verheyen, K.; Flinn, K.M.; Jacquemyn, H.; Kolb, A.; Van Calster, H.; Peterken, G.; Graae, B.J.; Bellemare, J.; Honnay, O.; et al. Homogenization of forest plant communities and weakening of species—Environment relationships via agricultural land use. J. Ecol. 2007, 95, 565–573. [Google Scholar] [CrossRef]
- Nowak, D.J. Contrasting natural regeneration and tree planting in fourteen North American cities. Urban For. Urban Green. 2012, 11, 374–382. [Google Scholar] [CrossRef]
- dos Anjos Santos, O.; Couceiro, S.R.M.; Rezende, A.C.C.; de Sousa Silva, M.D. Composition and richness of woody species in riparian forests in urban areas of Manaus, Amazonas, Brazil. Landsc. Urban Plan. 2016, 150, 70–78. [Google Scholar] [CrossRef]
- Homer, C.; Dewitz, J.; Yang, L.; Jin, S.; Danielson, P.; Xian, G.; Coulston, J.; Herold, N.; Wickham, J.; Megown, K. Completion of the 2011 National Land Cover Database for the Conterminous United States—Representing a Decade of Land Cover Change Information. Photogramm. Eng. Remote Sens. 2015, 81, 345–354. [Google Scholar]
- Landenberger, R.E.; Warner, T.A.; McGraw, J.B. Spatial patterns of female ailanthus altissima across an urban-to-rural land use gradient. Urban Ecosyst. 2009, 12, 437–448. [Google Scholar] [CrossRef]
- Huggett, R.; Wear, D.N.; Li, R.; Coulston, J.W.; Liu, S. Forecasts of Forest Conditions. In The Southern Forest Futures Project; Technical Report; Wear, D.N., Greis, J.G., Eds.; USDA Forest Service: Washington, DC, USA, 2013; pp. 73–102. [Google Scholar]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Stevens, G.C. The Elevational Gradient in Altitudinal Range: An Extension of Rapoport’s Latitudinal Rule to Altitude. Am. Nat. 2012, 140, 893–911. [Google Scholar] [CrossRef] [PubMed]







| Locality | Location Center | Ecological Province | Average Annual Rainfall (cm) | Urban Population Density (Person/km2) | Forest Type | Elevation Range (m) | Area Sampled (ha) | Number of Treed Plots | Number of Trees |
|---|---|---|---|---|---|---|---|---|---|
| Abingdon, VA | 36°42′35″ N, 81°58′32″ W | CABF | 116 | 392 | Urban | 590–709 | 1.56 | 39 | 279 |
| Periurban | 414–1419 | 3.83 | 57 | 1970 | |||||
| Atlanta, GA | 33°45′18″ N, 84°23′24″ W | SMF | 126 | 1218 | Urban | 159–331 | 11.92 | 298 | 1548 |
| Periurban | 145–513 | 2.02 | 30 | 659 | |||||
| Charlottesville, VA | 38°1′47.64″ N, 78°28′44.4″ W | CABF | 108 | 1640 | Urban | 92–198 | 2.36 | 59 | 409 |
| Periurban | 68–964 | 3.16 | 47 | 1205 | |||||
| East Orlando, FL | 28°35′33.54′′ N, 81°12′0.34′′ W | OCP | 129 | 1023 | Urban | 1–29 | 3.16 | 79 | 732 |
| Periurban | −1–39 | 1.75 | 26 | 603 | |||||
| Falls Church, VA | 38°52′56″ N, 77°10′16″ W | SMF | 113 | 2382 | Urban | 82–134 | 1.24 | 31 | 178 |
| Periurban | −25–174 | 1.28 | 19 | 357 | |||||
| Gainesville, FL | 29°39′7.19″ N, 82°19′29.97″ W | OCP | 120 | 783 | Urban | 15–61 | 2.04 | 51 | 659 |
| Periurban | 11–61 | 3.43 | 51 | 1429 | |||||
| Roanoke, VA | 37°16′0″ N, 79°56′0″ W | CABF | 105 | 880 | Urban | 271–531 | 4.56 | 114 | 1627 |
| Periurban | 234–1197 | 2.76 | 41 | 1044 | |||||
| Winchester, VA | 39°11′0″ N, 78°10′0″ W | CABF | 97 | 1096 | Urban | 194–287 | 2.24 | 56 | 336 |
| Periurban | 92–864 | 1.95 | 29 | 633 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blood, A.; Starr, G.; Escobedo, F.; Chappelka, A.; Staudhammer, C. How Do Urban Forests Compare? Tree Diversity in Urban and Periurban Forests of the Southeastern US. Forests 2016, 7, 120. https://doi.org/10.3390/f7060120
Blood A, Starr G, Escobedo F, Chappelka A, Staudhammer C. How Do Urban Forests Compare? Tree Diversity in Urban and Periurban Forests of the Southeastern US. Forests. 2016; 7(6):120. https://doi.org/10.3390/f7060120
Chicago/Turabian StyleBlood, Amy, Gregory Starr, Francisco Escobedo, Art Chappelka, and Christina Staudhammer. 2016. "How Do Urban Forests Compare? Tree Diversity in Urban and Periurban Forests of the Southeastern US" Forests 7, no. 6: 120. https://doi.org/10.3390/f7060120
APA StyleBlood, A., Starr, G., Escobedo, F., Chappelka, A., & Staudhammer, C. (2016). How Do Urban Forests Compare? Tree Diversity in Urban and Periurban Forests of the Southeastern US. Forests, 7(6), 120. https://doi.org/10.3390/f7060120