Enhancing Stand Structure through Snag Creation in Northeastern U.S. Forests: Using Ethanol Injections and Bark Beetle Pheromones to Artificially Stress Red Maple and White Pine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site
2.2. Ethanol Injections
2.3. Traps
2.4. Tree Surveys
2.5. Chlorophyll Fluorescence
3. Results
3.1. Beetles on White Pine
3.2. Beetles on Red Maple
3.3. Tree Surveys
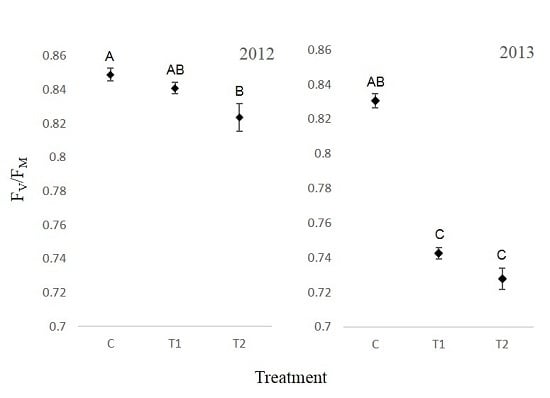
3.4. Chlorophyll Fluorescence
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thompson, J.R.; Carpenter, D.N.; Cogbill, C.V.; Foster, D.R. Four Centuries of Change in Northeastern United States Forests. PLoS ONE 2013, 8, e72540. [Google Scholar] [CrossRef] [PubMed]
- Schulte, L.A.; Mladenoff, D.J.; Crow, T.R.; Merrick, L.C.; Cleland, D.T. Homogenization of northern U.S. Great Lakes forests due to land use. Landsc. Ecol. 2007, 22, 1089–1103. [Google Scholar] [CrossRef]
- Fuller, T.L.; Foster, D.R.; McLachlan, T.S.; Drake, N. Impact of human activity on regional forest composition and dynamics in central New England. Ecosystems 1998, 1, 76–95. [Google Scholar] [CrossRef]
- Rhemtulla, J.M.; Mladenoff, D.J.; Clayton, M.K. Legacies of historical land use on regional forest composition and structure in Wisconsin, USA (mid-1800s-1930s-2000s). Ecol. Appl. 2009, 19, 1061–1078. [Google Scholar] [CrossRef] [PubMed]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.V.; Lattin, J.D.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.R.; et al. Ecology of Coarse Woody Debris in Temperate Ecosystems. Adv. Ecol. Res. 1986, 15, 133–302. [Google Scholar]
- Kilham, L. Reproductive behavior of hairy woodpeckers I. Pair Information and courtship. Wilson Bull. 1966, 78, 251–265. [Google Scholar]
- McComb, W.; Lindenmayer, D. Dying, dead and down trees. In Maintaining Biodiversity in Forest Ecosystems; Hunter, M.L., Jr., Ed.; Cambridge University Press: Cambridge, UK, 1999; pp. 335–372. [Google Scholar]
- Larson, M.J.; Jurgensen, M.F.; Harvey, A.E. N2 fixation associated with wood decayed by some common fungi in western Montana. Can. J. For. Res. 1978, 8, 341–345. [Google Scholar] [CrossRef]
- Evans, K.E.; Conner, R.N. Snag Management; General Technical Report NC-51; Department of Agriculture Forest Service: St. Paul, MN, USA, 1979; pp. 214–225. [Google Scholar]
- Hardt, R.A.; Swank, W.T. A comparison of structural and compositional characteristics of southern Appalachian young second-growth, maturing second-growth, and old-growth stands. Nat. Areas J. 1997, 17, 42–52. [Google Scholar]
- Duvall, M.D.; Grigal, D.F. Effects of timber harvesting on coarse woody debris in red pine forests across the Great Lakes states, USA. Can. J. For. Res. 1999, 29, 1926–1934. [Google Scholar] [CrossRef]
- McGee, G.G.; Leopold, D.J.; Nyland, R.D. Structural characteristics of old-growth, maturing, and partially cut northern hardwood forests. Ecol. Appl. 1999, 9, 1316–1329. [Google Scholar] [CrossRef]
- Cline, S.P.; Berg, A.B.; Wight, H.M. Snag characteristics and dynamics in Douglas-fir forests, Western Oregon. J. Wildl. Manag. 1980, 44, 773–786. [Google Scholar] [CrossRef]
- Siitonen, J. Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecol. Bull. 2001, 49, 11–41. [Google Scholar]
- Bull, E.L. The Value of Coarse Woody Debris to Vertebrates in the Pacific Northwest. In Proceedings of the Symposium on the Ecology and Management of Dead Wood in Western Forests, Reno, NV, USA, 2–4 November 2002; Laudenslayer, W.F., Jr., Shea, P.J., Valentine, B.E., Weatherspoon, C.P., Lisle, T.E., Eds.; Pacific Southwest Research Station: Reno, NV, USA, 2002; pp. 171–178. [Google Scholar]
- Bull, E.L.; Heater, T.W. Resting and denning sites of American martens in northeastern Oregon. Northwest Sci. 2000, 74, 179–185. [Google Scholar]
- Scott, V.E.; Evans, K.E.; Patton, D.R.; Stone, C.P. Cavity-Nesting Birds of North American Forests; U.S. Department of Agriculture: Cavity Nesting Birds of North American Forests; U.S. Department of Agriculture: Washington, DC, USA, 1977; Volume 511, p. 112.
- Boddy, L. Fungal Community Ecology and Wood Decomposition Processes in Angiosperms: From Standing Tree to Complete Decay of Coarse Woody Debris. Ecol. Bull. 2001, 43–56. [Google Scholar]
- Nappi, A.; Drapeau, P.; Leduc, A. How important is dead wood for woodpeckers foraging in eastern North American boreal forests? For. Ecol. Manag. 2015, 346, 10–21. [Google Scholar] [CrossRef]
- Stokland, J.N. Wood decomposition. In Biodiversity in Dead Wood; Stokland, J.N., Siitonen, J., Jonsson, B.G., Eds.; Cambridge University Press: New York, NY, USA, 2012; pp. 10–28. [Google Scholar]
- Kimmerer, T.W.; Kozlowski, T.T. Ethylene, Ethane, Acetaldehyde, and Ethanol Production By Plants under Stress. Plant Physiol. 1982, 69, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Moeck, H.A. Ethanol as the primary attractant for the ambrosia beetle Trypodendron lineatum (coleoptera: scolytidae). Can. Entomol. 1970, 102, 985–995. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Persad, A.B.; Herms, D.A. Ability of stress-related volatiles to attract and induced attacks by Xylosandrus germanus (Coleoptera: Curculionidae, Scolytinae) and other ambrosia beetles. Agric. For. Entomol. 2010, 12, 177. [Google Scholar] [CrossRef]
- Buchanan, W.D. Experiments with an ambrosia beetle, Xylosandrus germanus (Blfd.). J. Econ. Entomol. 1941, 34, 367–369. [Google Scholar] [CrossRef]
- Reding, M.E.; Oliver, J.B.; Schultz, P.B.; Ranger, C.M.; Youssef, N.N. Ethanol Injection of Ornamental Trees Facilitates Testing Insecticide Efficacy Against Ambrosia Beetles (Coleoptera: Curculionidae: Scolytinae). J. Econ. Entomol. 2013, 106, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Ranger, C.M.; Reding, M.E.; Oliver, J.B.; Schultz, P.B.; Moyseenko, J.J.; Youssef, N. Comparing efficacy of plant-derived essential oils for managing ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) and their corresponding mass spectral characterization. J. Econ. Entomol. 2011, 104, 1665. [Google Scholar] [CrossRef] [PubMed]
- Ranger, C.M.; Reding, M.E.; Schultz, P.B.; Oliver, J.B. Ambrosia beetle (Coleoptera: Curculionidae) responses to volatile emissions associated with ethanol-injected Magnolia virginiana L. Environ. Entomol. 2012, 41, 636. [Google Scholar] [CrossRef] [PubMed]
- Bull, E.L.; Partridge, A.D. Methods of killing trees for use by cavity nesters. Wildl. Soc. Bull. 1986, 14, 142–146. [Google Scholar]
- Filip, G.M.; Parks, C.G.; Baker, F.A.; Daniels, S.E. Artificial inoculation of decay fungi into Douglas-Fir with rifle or shotgun to produce wildlife trees in western Oregon. West. J. Appl. For. 2004, 19, 211–215. [Google Scholar]
- Baker, F.A.; Daniels, S.E.; Parks, C.A. Technical Notes: Inoculating Trees with Wood Decay Fungi with Rifle and Shotgun. West. J. Appl. For. 1996, 11, 13–15. [Google Scholar]
- Ross, D.W.; Niwa, C.G. Using aggregation and antiaggregation pheromones of the Douglas-fir beetle to produce snags for wildlife habitat. West. J. Appl. For. 1997, 12, 52–54. [Google Scholar]
- Kelsey, R.G. Chemical Indicators of Stress in Trees: Their Ecological Significance and Implication for Forestry in Eastern Oregon and Washington. Northwest Sci. 2001, 75, 70–76. [Google Scholar]
- Kelsey, R.G.; Gallego, D.; Sánchez-García, F.J.; Pajares, J.A. Ethanol accumulation during severe drought may signal tree vulnerability to detection and attack by bark beetles. Can. J. For. Res. 2014, 44, 554–561. [Google Scholar] [CrossRef]
- Peñuelas, J.; Munné-Bosch, S. Isoprenoids: An evolutionary pool for photoprotection. Trends Plant Sci. 2005, 10, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Rosenqvist, E.; van Kooten, O. Chlorophyll Fluorescence: A General Description and Nomenclature. In Practical Applications of Chlorophyll Fluorescence in Plant Biology; DeEll, J., Toivonen, P.A., Eds.; Springer US: New York, NY, USA, 2003; pp. 31–77. [Google Scholar]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Zielinski, R.E.; Zangerl, A.R.; Crofts, A.R.; Berenbaum, M.R.; DeLucia, E.H. The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera: Noctuidae) on photosynthesis in Arabidopsis thaliana. J. Exp. Bot. 2006, 57, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Zangerl, A.R.; Hamilton, J.G.; Miller, T.J.; Crofts, A.R.; Oxborough, K.; Berenbaum, M.R.; de Lucia, E.H. Impact of folivory on photosynthesis is greater than the sum of its holes. Proc. Natl. Acad. Sci. USA 2002, 99, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Govindjee, A.S. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the fluorescence transient. In Chlorophyll Fluorescence: A Signature of Photosynthesis; Dordrecht, S., Ed.; Advances in Photosynthesis and Respiration Series; University of Geneva: Geneva, Switzerland, 2004; pp. 321–362. [Google Scholar]
- Allison, J.D.; Borden, J.H.; McIntosh, R.L.; de Groot, P.; Gries, R. Kairomonal response by four Monochamus species (Coleoptera : Cerambycidae) to bark beetle pheromones. J. Chem. Ecol. 2001, 27, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Asaro, C.; Crowe, C.M.; Duerr, D.A. Bark beetle pheromones and pine volatiles: Attractant kairomone lure blend for longhorn beetles (Cerambycidae) in pine stands of the southeastern United States. J. Econ. Entomol. 2011, 104, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.; Nott, R.W. Response of the whitespotted sawyer beetle, Monochamus s. scutellatus, and associated woodborers to pheromones of some Ips and Dendroctonus bark beetles. J. Appl. Entomol. 2004, 128, 483–487. [Google Scholar] [CrossRef]
- Wood, S.L. The Bark and Ambrosia Beetles of North and Central America (Coleoptera: Scolytidae), a Taxonomic Monograph. Great Basin Nat. Mem. 1982, 6, 1–1356. [Google Scholar]
- Lingafelter, S.W. Illustrated Key to the Longhorned Woodboring Beetles of the Eastern United States; Coleopterists Society: North Potomac, MD, USA, 2007; p. 206. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for eduation and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Kautsky, H.; Hirsch, A. Neue Versuche zur Kohlensäureassimilation. Naturwissenschaften 1931, 19, 964. [Google Scholar] [CrossRef]
- Miller, D.R.; Asaro, C.; Berisford, C.W. Attraction of southern pine engravers and associated bark beetles (Coleoptera: Scolytidae) to ipsenol, ipsdienol, and lanierone in southeastern United States. J. Econ. Entomol. 2005, 98, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Schenk, J.A.; Benjamin, D.M. A tentative classification of jack pine susceptible to bark beetle attack in central Wisconsin. J. For. 1964, 62, 570–574. [Google Scholar]
- Kegley, S.J.; Livingston, R.L.; Gibson, K.E. Pine Engraver, Ips pini (Say), in the Western United States; U.S. Department of Agriculture, F.S., Ed.; U.S. Department of Agriculture, Forest Service, Cooperative Forestry and Forest Health: Missoula, MT, USA, 1997.
- Gara, R.I.; Millegan, D.R.; Gibson, K.E. Integrated pest management of Ips pini (Col., Scolytidae) populations in south-eastern Montana. J. Appl. Entomol. Z. Angew. Entomol. 1999, 123, 529–534. [Google Scholar] [CrossRef]
- Klepzig, K.D.; Raffa, K.F.; Smalley, E.B. Association of an insect-fungal complex with red pine decline in Wisconsin. For. Sci. 1991, 37, 1119–1139. [Google Scholar]
- Ryall, K.L.; de Groot, P.; Smith, S.M. Sequential patterns of colonization of coarse woody debris by Ips pini (Say) (Coleoptera: Scolytidae) following a major ice storm in Ontario. Agric. For. Entomol. 2006, 8, 89–95. [Google Scholar] [CrossRef]
- Miller, D.R.; Dodds, K.J.; Eglitis, A.; Fettig, C.J.; Hofstetter, R.W.; Langor, D.W.; Mayfield, A.E.; Munson, A.S.; Poland, T.M.; Raffa, K.F. Trap lure blend of pine volatiles and bark beetle pheromones for Monochamus spp. (Coleoptera: Cerambycidae) in Pine Forests of Canada and the United States. J. Econ. Entomol. 2013, 106, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Chenier, J.V.R.; Philogene, B.J.R. Field responses of certain forest Coleoptera to conifer monoterpenes and ethanol. J. Chem. Ecol. 1989, 15, 1729–1746. [Google Scholar] [CrossRef] [PubMed]
- Petrice, T.R.; Haack, R.A.; Poland, T.M. Evaluation of three trap types and five lures for monitoring Hylurgus ligniperda (Coleoptera: Scolytidae) and other local scolytids in New York. Great Lakes Entomol. 2004, 37, 1–9. [Google Scholar]
- Sun, J.H.; Miao, Z.W.; Zhang, Z.; Zhang, Z.N.; Gillette, N.E. Red turpentine beetle, Dendroctonus valens LeConte (Coleoptera : Scolytidae), response to host semiochemicals in China. Environ. Entomol. 2004, 33, 206–212. [Google Scholar] [CrossRef]
- Raffa, K.F.; Phillips, T.W.; Salom, S.M. Strategies and Mechanisms of Host Colonization by Bark Beetles; Academic: San Diego, CA, USA, 1993; pp. 102–128. [Google Scholar]
- Miller, D.R.; Rabaglia, R.J. Ethanol and (-)-alpha-pinene: Attractant kairomones for bark and ambrosia beetles in the southeastern US. J. Chem. Ecol. 2009, 35, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Ploetz, R.C.; Hulcr, J.; Wingfield, M.J.; de Beer, Z.W. Destructive Tree Diseases Associated with Ambrosia and Bark Beetles: Black Swan Events in Tree Pathology? Plant Dis. 2013, 97, 856–872. [Google Scholar] [CrossRef]
- Reding, M.; Oliver, J.; Schultz, P.; Ranger, C. Monitoring flight activity of ambrosia beetles in ornamental nurseries with ethanol-baited traps: Influence of trap height on captures. J. Environ. Hortic. 2010, 28, 85–90. [Google Scholar]
- Dodds, K.J.; Miller, D.R. Test of nonhost angiosperm volatiles and verbenone to protect trap trees for Sirex noctilio (Hymenoptera: Siricidae) from attacks by bark beetles (Coleoptera: Scolytidae) in the northeastern United States. J. Econ. Entomol. 2010, 103, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Dodds, K.J.; Zylstra, K.E.; Dubois, G.D.; Hoebeke, E.R. Arboreal insects associated with herbicide-stressed Pinus resinosa and Pinus sylvestris used as Sirex noctilio trap trees in New York. Environ. Entomol. 2012, 41, 1350–1363. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, K.S.; Steele, T.W. Snag dynamics in northern hardwood forests under different management scenarios. For. Ecol. Manag. 2016, 363, 267–276. [Google Scholar] [CrossRef]
- Ranius, T.; Caruso, A.; Jonsell, M.; Juutinen, A.; Thor, G.; Rudolphi, J. Dead wood creation to compensate for habitat loss from intensive forestry. Biol. Conserv. 2014, 169, 277–284. [Google Scholar] [CrossRef]
- Zylstra, K.E.; Dodds, K.J.; Francese, J.A.; Mastro, V.C. Sirex noctilio (Hymenoptera: Siricidae) in North America: The effect of stem-injection timing on the attractiveness and suitability of trap trees. Agric. For. Entomol. 2010, 12, 243–250. [Google Scholar]






| Control | Single ETOH Injection | Double ETOH Injections | P-Value | |
|---|---|---|---|---|
| Total Scolytinae and Cerambycidae | 66 ± 9.9 B | 318 ± 45.6 A | 322 ± 30.2 A | 0.0001 |
| Total Scolytinae | 61.4 ± 9.4 B | 297.2 ± 43.4 A | 300.8 ± 27.1 A | 0.0001 |
| Crypturgus borealis | 2.4 ± 0.4 B | 3.8 ± 1.2 B | 10.0 ± 2.7 A | 0.022 |
| Dendroctonus valens | 2.0 ± 0.95 | 5.0 ± 2.6 | 7.2 ± 1.5 | 0.1706 |
| Gnathotrichus materiarius | 15.2 ± 3.1 | 13.6 ± 2.6 | 17.8 ± 6.9 | 0.8265 |
| Hylastes opacus | 5.0 ± 2.5 | 5.0 ± 2.2 | 3.6 ± 1.5 | 0.8613 |
| Ips calligraphus | 0.0 ± 0.0 | 3.2 ± 1.0 | 28.6 ± 14.4 | 0.0624 |
| Ips grandicollis | 0.8 ± 0.4 B | 23.6 ± 3.8 A | 27.8 ± 7.9 A | 0.0056 |
| Ips pini | 0.4 ± 0.4 B | 190.2 ± 33.1 A | 114.8 ± 25.6 A | 0.0005 |
| Orthotomicus caelatus | 9.8 ±2.7 B | 21 ± 6.6 AB | 54.2 ± 14.5 A | 0.0147 |
| Pityogenes. hopkinsi | 8.4 ± 1.7 | 9.0 ± 2.8 | 7.6 ± 1.1 | 0.8844 |
| Xyloterinus politus | 2.6 ± 0.7 | 5.2 ± 1.8 | 3.8 ± 1.6 | 0.4661 |
| Total Cerambycidae | 5.4 ± 1.6 B | 20.8 ± 2.6 A | 21.8 ± 3.9 A | 0.0022 |
| Monochamus notatus | 0.0 ± 0.0 B | 4.0 ± 1.4 AB | 8.0 ± 1.6 A | 0.0024 |
| Control | Single ETOH Injection | Double ETOH Injections | P-Value | |
|---|---|---|---|---|
| Total Scolytinae and Cerambycidae | 41.4 ± 4.5 | 68.0 ± 26.1 | 75.6 ± 14.3 | 0.4 |
| Total Scolytinae | 36.6 ± 3.5 | 64.6 ± 25.2 | 68.8 ± 12.6 | 0.35 |
| Gnathotrichus materiarius | 3.6 ± 1.2 | 8.8 ± 3.6 | 11.0 ± 3.6 | 0.25 |
| Hylastes opacus | 2.4 ± 0.5 | 4.2 ± 1.8 | 3.6 ± 0.9 | 0.58 |
| Monarthrum mali | 0.6 ± 0.4 | 8.0 ± 4.3 | 5.6 ± 3.5 | 0.29 |
| Orthotomicus caelatus | 5.0 ± 0.5 | 5.2 ± 2.4 | 7.2 ± 2.0 | 0.65 |
| Pityogenes hopkinsi | 6.2 ± 1.2 | 5.0 ± 2.5 | 6.0 ± 1.8 | 0.89 |
| Xyloterinus politus | 7.0 ± 1.1 | 18.6 ± 7.8 | 14.2 ± 2.4 | 0.26 |
| Total Cerambycidae | 4.8 ± 1.5 | 3.4 ± 1.2 | 6.8 ± 3.1 | 0.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodds, K.J.; Hanavan, R.P.; Wansleben, T. Enhancing Stand Structure through Snag Creation in Northeastern U.S. Forests: Using Ethanol Injections and Bark Beetle Pheromones to Artificially Stress Red Maple and White Pine. Forests 2016, 7, 124. https://doi.org/10.3390/f7060124
Dodds KJ, Hanavan RP, Wansleben T. Enhancing Stand Structure through Snag Creation in Northeastern U.S. Forests: Using Ethanol Injections and Bark Beetle Pheromones to Artificially Stress Red Maple and White Pine. Forests. 2016; 7(6):124. https://doi.org/10.3390/f7060124
Chicago/Turabian StyleDodds, Kevin J., Ryan P. Hanavan, and Tom Wansleben. 2016. "Enhancing Stand Structure through Snag Creation in Northeastern U.S. Forests: Using Ethanol Injections and Bark Beetle Pheromones to Artificially Stress Red Maple and White Pine" Forests 7, no. 6: 124. https://doi.org/10.3390/f7060124
APA StyleDodds, K. J., Hanavan, R. P., & Wansleben, T. (2016). Enhancing Stand Structure through Snag Creation in Northeastern U.S. Forests: Using Ethanol Injections and Bark Beetle Pheromones to Artificially Stress Red Maple and White Pine. Forests, 7(6), 124. https://doi.org/10.3390/f7060124