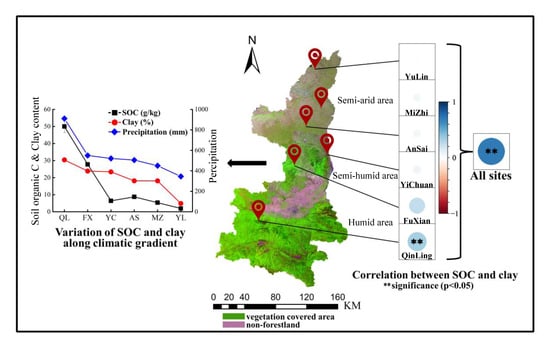
Relationship between Soil Organic Carbon Stocks and Clay Content under Different Climatic Conditions in Central China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas and Experimental Design
2.2. Soil Sampling
2.3. Soil Analysis
2.4. Vegetation Diversity
2.5. Data Analysis
3. Results
3.1. Changes in Soil Properties
3.2. Changes in Vegetation Diversity along the Rainfall Gradient
3.3. Relationship between Vegetation Characteristics, Soil PSD, and Soil Chemical Properties
4. Discussion
4.1. Relationship between Clay and SOC Depended on Precipitation in Different Climate Types
4.2. Relationship between Clay and SOC Depended on Vegetation and Soil Properties in Same Climate Type
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; de Remy de Courcelles, V.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Wang, G.; Qian, J.; Cheng, G.; Lai, Y. Soil organic carbon pool of grassland soils on the Qinghai-Tibetan Plateau and its global implication. Sci. Total Environ. 2002, 291, 207–217. [Google Scholar]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruba, P.; Socha, J.; Błońska, E.; Lasota, J. Effect of variable soil texture, metal saturation of soil organic matter (SOM) and tree species composition on spatial distribution of SOM in forest soils in Poland. Sci. Total Environ. 2015, 521–522, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Gonsalves, D.R.P.; de Moraes Sá, J.C.; Mishra, U.; Cerri, C.E.P.; Ferreira, L.A.; Furlan, F.J.F. Soil type and texture impacts on soil organic carbon storage in a sub-tropical agro-ecosystem. Geoderma 2017, 286, 88–97. [Google Scholar] [CrossRef]
- Johannes, A.; Matter, A.; Schulin, R.; Weisskopf, P.; Baveye, P.C.; Boivin, P. Optimal organic carbon values for soil structure quality of arable soils. Does clay content matter? Geoderma 2017, 302, 14–21. [Google Scholar] [CrossRef]
- Jagadamma, S.; Lal, R. Distribution of organic carbon in physical fractions of soils as affected by agricultural management. Biol. Fertil. Soils 2010, 46, 543–554. [Google Scholar] [CrossRef]
- Giardina, C.P.; Ryan, M.G.; Hubbard, R.M.; Binkley, D. Tree species and soil textural controls on carbon and nitrogen mineralization rates. Soil Sci. Soc. Am. J. 2001, 65, 1272–1279. [Google Scholar] [CrossRef]
- Schillaci, C.; Acutis, M.; Lombardo, L.; Lipani, A.; Fantappiè, M.; Märker, M.; Saia, S. Spatio-temporal topsoil organic carbon mapping of a semi-arid Mediterranean region: The role of land use, soil texture, topographic indices and the influence of remote sensing data to modelling. Sci. Total Environ. 2017, 601–602, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shi, Z.; Li, D.; Rey, A.; Ruan, H.; Craine, J.M.; Liang, J.; Zhou, J.; Luo, Y. Soil properties control decomposition of soil organic carbon: Results from dataassimilation analysis. Geoderma 2016, 262, 235–242. [Google Scholar] [CrossRef]
- Saiz, G.; Bird, M.I.; Domingues, T.; Schrodt, F.; Schwarz, M.; Feldpausch, T.R.; Veenendaal, E.; Djagbletey, G.; Hien, F.; Compaoré, H.; et al. Variation in soil carbon stocks and their determinants across a precipitation gradient in West Africa. Glob. Chang. Biol. 2012, 18, 1670–1683. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Barthold, F.; Blank, B.; Kogel-Knabner, I. Digital mapping of soil organic matter stocks using Random Forest modeling in a semi-arid steppe ecosystem. Plant Soil 2011, 340, 7–24. [Google Scholar] [CrossRef]
- Goebel, M.O.; Bachmann, J.; Reichstein, M.; Janssens, I.A.; Guggenberger, G. Soil water repellency and its implications for organic matter decomposition-is there a link to extreme climatic events? Glob. Chang. Biol. 2011, 17, 2640–2656. [Google Scholar] [CrossRef]
- Shakesby, R.A.; Doerr, S.H.; Walsh, R.P.D. The erosional impact of soil hydrophobicity: Current problems and future research directions. J. Hydrol. 2000, 231, 178–191. [Google Scholar] [CrossRef]
- Pardini, G.; Gispert, M.; Dunjó, G. Runoff erosion and nutrient depletion in five Mediterranean soils of NE Spain under different land use. Sci. Total Environ. 2003, 309, 213–224. [Google Scholar] [CrossRef]
- Hassink, J. Preservation of plant residues in soils differing in Unsaturated Protective Capacity. Soil Sci. Soc. Am. J. 1996, 60, 487–491. [Google Scholar] [CrossRef]
- Hassink, J. The capacity of soils to preserve organic C and N by their association with clay and silt particles. Plant Soil 1997, 191, 77–87. [Google Scholar] [CrossRef]
- Feng, W.T.; Plante, A.F.; Six, J. Improving estimates of maximal organic carbon stabilization by fine soil particles. Biogeochemistry 2013, 112, 81–93. [Google Scholar] [CrossRef]
- Jin, Z.; Dong, Y.S.; Qi, Y.C.; Liu, W.G.; An, Z.S. Characterizing variations in soil particle-size distribution along a grass-desert shrub transition in the Ordos Plateau of Inner Mongolia, China. Land Degrad. Dev. 2013, 24, 141–146. [Google Scholar] [CrossRef]
- Gao, G.L.; Ding, G.D.; Zhao, Y.Y.; Wu, B.; Zhang, Y.Q.; Qin, S.G.; Bao, Y.F.; Yu, M.H.; Liu, Y.D. Fractal approach to estimating changes in soil properties following the establishment of Caragana Korshinskii shelterbelts in Ningxia, NW China. Ecol. Indic. 2014, 43, 236–243. [Google Scholar] [CrossRef]
- Deng, Y.S.; Cai, C.F.; Xia, D.; Ding, S.W.; Chen, J.Z. Fractal features of soil particle size distribution under different land-use patterns in the alluvial fans of collapsing gullies in the hilly granitic region of southern China. PLoS ONE 2017, 12, e0173555. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.J.; Chen, J.; Deng, J.; Zhao, F.Z.; Han, X.H.; Yang, G.H.; Tong, X.G.; Feng, Y.Z.; Shelton, S.; Ren, G.X. Response of microbial diversity to C:N:P stoichiometry in fine root and microbial biomass following afforestation. Biol. Fertil. Soils 2017, 53, 457–468. [Google Scholar] [CrossRef]
- Martens, D.A.; Reedy, T.E.; Lewis, D.T. Soil organic carbon content and composition of 130-year crop, pasture and forest land-use managements. Glob. Chang. Biol. 2004, 10, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Nepstad, D.C.; Carvalho, C.R.; Davidson, E.A.; Jipp, P.; Lefebvre, P.; Negreiros, G.H.D.; da Silva, E.D.; Stone, T.A.; Trumbore, S.; Vieira, S. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 1994, 372, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Angers, D.A.; Caron, J. Plant-induced changes in soil structure: Processes and feedbacks. Biogeochemistry 1998, 42, 55–72. [Google Scholar] [CrossRef]
- Rodriguez-Lado, L.; Lado, M. Relation between soil forming factors and scaling properties of particle size distributions derived from multifractal analysis in topsoils from Galicia (NW Spain). Geoderma 2017, 287, 147–156. [Google Scholar] [CrossRef]
- Tracy, S.R.; Black, C.R.; Roberts, J.A.; Mooney, S.J. Exploring the interacting effect of soil texture and bulk density on root system development in tomato (Solanum lycopersicum L.). Environ. Exp. Bot. 2013, 91, 38–47. [Google Scholar] [CrossRef]
- Han, X.H. Evaluation and Ecological Effects of Returning Farmland to Forest in Loess Hilly and Gully Region; Science Press: Beijing, China, 2018. [Google Scholar]
- Sang, G.S. Vegetation variation of Loess Plateau during human history. J. Arid Land Resour. Environ. 2005, 19, 54–58. (In Chinese) [Google Scholar]
- Yi, L.; Ren, Z.Y.; Zhang, C.; Liu, W. Vegetation cover, climate and human activities on the Loess Plateau. Resour. Sci. 2014, 36, 166–174. (In Chinese) [Google Scholar]
- Food Agriculture Organization (FAO). Soil Map of the World. Nature 1957, 179, 1168. [Google Scholar] [CrossRef]
- Mariotte, C.A.; Hudson, G.; Hamilton, D.; Neilson, R.; Boag, B.; Handley, L.L.; Wishart, J.; Scrimgeour, C.M.; Robinson, D. Spatial variability of soil total C and N and their stable isotopes in an upland Scottish grassland. Plant Soil 1997, 196, 151–162. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Gong, Y.M.; Wang, X.; Hu, Y.K. Volume fractal dimension of soil particles and relationships with soil physical-chemical properties and plant species diversity in an alpine grassland under different disturbance degrees. J. Arid Land 2013, 5, 480–487. [Google Scholar] [CrossRef]
- Lu, N.; Fu, B.; Jin, T.; Chang, R. Trade-off analyses of multiple ecosystem services by plantations along a precipitation gradient across Loess Plateau landscapes. Landsc. Ecol. 2014, 29, 1697–1708. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, 2nd ed.; Page, A.L., Keeney, D.R., Baker, D.E., Miller, R.H., Ellis, R., Jr., Rhoades, J.D., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; Part 2; pp. 539–579. [Google Scholar]
- Havlin, J.L.; Kissel, D.E.; Maddux, L.D.; Claassen, M.M.; Long, J.H. Crop rotation and tillage effects on soil organic carbon and nitrogen. Soil Sci. Soc. Am. J. 1990, 54, 448–452. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, Z.B.; Li, P. Particle fractal dimension and total phosphorus of soil in a typical watershed of Yangtze River, China. Environ. Earth Sci. 2015, 73, 6091–6099. [Google Scholar] [CrossRef]
- Liu, E.K.; Yan, C.R.; Mei, X.R.; He, W.Q.; Bing, S.H.; Ding, L.P.; Liu, Q.; Liu, S.; Fan, T.L. Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Ma, K.P.; Liu, Y.M. Measurement of biotic community diversity. I. α diversity (Part 2). Chin. Biodivers. 1994, 2, 162–168. (In Chinese) [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Mechanisms of carbon sequestration in soil aggregates. Crit. Rev. Plant Sci. 2004, 23, 481–504. [Google Scholar] [CrossRef]
- Niu, D.F.; Li, B.S.; Wang, F.N.; Wen, X.H.; Ma, J.L.; Shu, P.X. Climate changes indicated by the clay minerals: A case of the Dishaogouwan section on the southeastern margin of the Mu Us Desret. J. Fuzhou Univ. (Nat. Sci. Ed.) 2015, 43, 345–351. [Google Scholar]
- Fang, J.Y.; Piao, S.L.; Tang, Z.Y.; Peng, C.H.; Wei, J. Interannual variability in net primary production and precipitation. Science 2001, 293, 1723. [Google Scholar] [CrossRef] [PubMed]
- Callesen, I.; Liski, J.; Raulund-Rasmussen, K.; Olsson, M.T.; Tau-Strand, L.; Vesterdal, L.; Westman, C.J. Soil carbon stores in Nordic well-drained forest soils-relationships with climate and texture class. Glob. Chang. Biol. 2003, 9, 358–370. [Google Scholar] [CrossRef]
- Deng, Q.; Cheng, X.L.; Hui, D.F.; Zhang, Q.; Li, M.; Zhang, Q.F. Soil microbial community and its interaction with soil carbon and nitrogen dynamics following afforestation in central China. Sci. Total Environ. 2016, 541, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.T. Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur. J. Soil Sci. 2001, 52, 345–353. [Google Scholar] [CrossRef]
- Denef, K.; Six, J.; Merckx, R.; Paustian, K. Short-term effects of biological and physical forces on aggregate formation in soils with different clay mineralogy. Plant Soil 2002, 246, 185–200. [Google Scholar] [CrossRef]
- Zaffar, M.; Lu, S.G. Pore Size Distribution of Clayey Soils and Its Correlation with Soil Organic Matter. Pedosphere 2015, 25, 240–249. [Google Scholar] [CrossRef]
- Chen, J.Q. Clay minerals of soils on the north slope of Taibai Mountain. Atca Pedol. Sin. 1982, 19, 273–282. (In Chinese) [Google Scholar]
- Zheng, H.H.; Theng, B.K.G.; Whitton, J.S. Mineral compsition of loess-paleosol in the Loess Plateau of China and its environmental implications. Geochimica 1994, 23, 113–123. (In Chinese) [Google Scholar]
- Jiang, J.P.; Xiong, Y.C.; Jiang, H.M.; Ye, D.Y.; Song, Y.J.; Li, F.M. Soil microbial activity during secondary vegetation succession in semiarid abandoned lands of Loess Plateau. Pedosphere 2009, 19, 735–747. [Google Scholar] [CrossRef]
- Burke, I.C.; Yonker, C.M.; Parton, W.J.; Cole, C.V.; Schimel, D.S.; Flash, K. Texture, Climate, and Cultivation Effects on Soil Organic Matter Content in U.S. Grassland Soils. Soil Sci. Soc. Am. J. 1989, 53, 800–805. [Google Scholar] [CrossRef]
- Gould, I.J.; Quinton, J.N.; Weigelt, A.; De Deyn, G.B.; Bardgett, R.D. Plant diversity and root traits benefit physical properties key to soil function in grasslands. Ecol. Lett. 2016, 19, 1140–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinoga, J.D.R.; Pariente, S.; Diaz, A.R.; Murillo, J.F.M. Variability of relationships between soil organic carbon and some soil properties in Mediterranean rangelands under different climatic conditions (South of Spain). Catena 2012, 94, 17–25. [Google Scholar] [CrossRef]
- Post, W.M.; Emanuel, W.R.; Zinke, P.J.; Stangenberger, A.G. Soil carbon pools and world life zones. Nature 1982, 298, 156–159. [Google Scholar] [CrossRef]
- Liu, L.; Gundersen, P.; Zhang, T.; Mo, J.M. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 2012, 44, 31–38. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E.; Bach, E.M.; Hofmockel, K.S.; Kazanski, C.E. Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry 2015, 125, 203–219. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Schmidt, S.K. Phosphorus limitation of microbial processes in moist tropical forests: Evidence from short-term laboratory incubations and field studies. Ecosystems 2002, 5, 680–691. [Google Scholar] [CrossRef]
- Gijsman, A.J.; Oberson, A.; Tiessen, H.; Friesen, D.K. Limited applicability of the CENTURY model to highly weathered tropical soils. Agron. J. 1996, 88, 894–903. [Google Scholar] [CrossRef]
- Augusto, L.; Delerue, F.; Gallet-Budynek, A.; Achat, D.L. Global assessment of limitation to symbiotic nitrogen fixation by phosphorus availability in terrestrial ecosystems using a meta-analysis approach. Glob. Biogeochem. Cycles 2013, 27, 804–815. [Google Scholar] [CrossRef] [Green Version]
- Gusewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Thiet, R.K.; Frey, S.D.; Six, J. Do growth yield efficiencies differ between soil microbial communities differing in fungal: Bacterial ratios? Reality check and methodological issues. Soil Biol. Biochem. 2006, 38, 837–844. [Google Scholar] [CrossRef]
- Cheng, F.; Peng, X.B.; Zhao, P.; Yuan, J.; Zhong, C.G.; Cheng, Y.L.; Cui, C.; Zhang, S.X. Soil microbial biomass, basal respiration and enzyme activity of main forest types in the Qinling Mountains. PLoS ONE 2013, 8, e67353. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.M.; Cao, J.; Wang, C.Y.; Wang, G. Microbial biomass and nutrients in soil at the different stages of secondary forest succession in Ziwulin, northwest China. For. Ecol. Manag. 2005, 217, 117–125. [Google Scholar] [CrossRef]






| Geographical Coordinates | Altitude (m) | Slope Gradient (°) | T (°C) | PH | SBD (g/cm3) | Soil Type a | ||
|---|---|---|---|---|---|---|---|---|
| 0–10 cm | 10–20 cm | |||||||
| QL | 33°59′58″~34°05′48″ N; 107°41′30″~107°48′36″ E | 2355 | 25–45 | 11.4 | 5.98 ± 0.03 d | 1.05 ± 0.00 e | 1.09 ± 0.00 f | Orthic Acrisols |
| FX | 35°59′39″~36°08′42″ N; 108°41′13″~109°40′45″ E | 1227 | 5–43 | 8.1 | 8.01 ± 0.08 c | 1.15 ± 0.00 d | 1.17 ± 0.00 e | Chromic Cambisols |
| YC | 36°04′48″~36°09′52″ N; 110°17′59″~110°18′33″ E | 875 | 0–12 | 10 | 8.25 ± 0.02 b | 1.17 ± 0.01 c | 1.21 ± 0.01 c | Chromic Cambisols |
| AS | 36°43′45″~36°54′36″ N; 109°15′16″~109°21′06″ E | 1237 | 12–45 | 8.8 | 8.38 ± 0.02 ab | 1.15 ± 0.00 d | 1.19 ± 0.00 d | Chromic Cambisols |
| MZ | 37°45′37″~37°51′47″ N; 110°10′48″~110°15′43″ E | 1071 | 18–30 | 8.5 | 8.51 ± 0.05 a | 1.21 ± 0.00 b | 1.24 ± 0.01 b | Calcic Cambisols |
| YL | 37°24′45″~38°28′33″ N; 109°35′42″~109°49′22″ E | 1151 | 5–35 | 10.7 | 8.39 ± 0.04 ab | 1.38 ± 0.00 a | 1.43 ± 0.00 a | Calcic Cambisols |
| Site | Climatic Conditions | Major Vegetation Types | TCD (%) | SCD (%) | GC (%) | Vegetation Community |
|---|---|---|---|---|---|---|
| QL | humid | native forest | 87.31 ± 3.03 a | 25.77 ± 5.03 b | 27.31 ± 8.50 c | Trees: Quercus liaotungensis Koidz; Quercus aliena var. acutiserrata; Larix chinensis Beissn; Abies fargesii Franch. |
| FX | sub-humid | secondary forest | 73.16 ± 2.63 b | 21.68 ± 3.40 bc | 39.21 ± 5.02 bc | Trees: Betula platyphylla Suk; Populus davidiana Dode; Pinus tabulaeformis Carr; Platycladus orientalis (L.) Franco; Quercus liaotungensis Koidz. Shurbs: Rhamnus utilis Decne; Acer ginnala Maxim; Celtis bungeana Bl. |
| YC | sub-humid | artificial vegetation | 60.00 ± 7.42 b | 5.20 ± 0.80 c | 48.00 ± 7.58 abc | Trees: Armeniaca sibirica (L.) Lam; Pinus tabulaeformis Carr; Robinia pseudoacacia Linn; Platycladus orientalis (L.) Franco. |
| AS | semi-arid | artificial vegetation | 56.88 ± 4.62 b | 43.75 ± 8.26 a | 65.06 ± 3.72 a | Trees: Robinia pseudoacacia Linn. Shurbs: Caragana korshinskii Kom. Grass: Artemisia vestita Wall; Astragalus melilotoides Pall; Carex hirta Linn; Stipa grandis P. Smirn. |
| MZ | semi-arid | artificial vegetation | 61.00 ± 9.11 b | 11.12 ± 2.27 bc | 63.00 ± 8.89 a | Trees: Populus simonii Carr; Pinus tabulaeformis Carr; Armeniaca sibirica (L.) Lam; Platycladus orientalis (L.) Franco; Robinia pseudoacacia Linn. |
| YL | semi-arid | desert scrub | 3.89 ± 0.83 c | 16.67 ± 5.21 bc | 61.11 ± 5.32 ab | Trees: Salix matsudana Koidz; Salix cheilophila Schneid. Shurb: Hedysarumscoparium Fisch; Artemisia ordosica Krasch. Grass: Artemisia desertorum Spreng; Amorpha fruticosa Linn. |
| Site | Clay (<2 μm) | Silt (50~2 μm) | Sand (2000~50 μm) |
|---|---|---|---|
| 0–10 cm | |||
| QL | 30.4 ± 0.71 a | 60.8 ± 0.57 a | 8.8 ± 0.90 e |
| FX | 23.9 ± 0.32 b | 62.3 ± 0.34 a | 13.9 ± 0.34 c |
| YC | 23.4 ± 0.63 b | 55.9 ± 1.22 b | 20.7 ± 1.76 d |
| AS | 18.1 ± 0.29 c | 45.3 ± 0.63 c | 36.5 ± 0.62 b |
| MZ | 18.1 ± 0.65 c | 47.1 ± 1.59 c | 34.8 ± 2.10 b |
| YL | 4.9 ± 0.37 d | 4.8 ± 1.15 d | 90.4 ± 1.49 a |
| 10–20 cm | |||
| QL | 30.4 ± 0.95 a | 56.8 ± 0.62 a | 12.8 ± 1.24 d |
| FX | 24.3 ± 0.20 b | 55.5 ± 0.65 ab | 20.3 ± 0.72 c |
| YC | 24.0 ± 0.12 b | 54.2 ± 0.74 b | 21.8 ± 0.78 c |
| AS | 18.5 ± 0.150 c | 49.3 ± 0.57 c | 32.2 ± 0.59 b |
| MZ | 18.4 ± 0.18 c | 48.2 ± 2.08 c | 33.5 ± 2.13 b |
| YL | 9.5 ± 0.44 d | 12.1 ± 0.21 d | 78.4 ± 0.36 a |
| Study sites | PSD | Stock of Soil Nutrients | Content of Soil Nutrients | ||||
|---|---|---|---|---|---|---|---|
| SOC | TN | TP | SOC | TN | TP | ||
| All sites | Clay | 0.649 ** | 0.655 ** | 0.397 ** | 0.648 ** | 0.658 ** | 0.467 ** |
| Silt | 0.500 ** | 0.507 ** | 0.552 ** | 0.491 ** | 0.501 ** | 0.589 ** | |
| Sand | −0.565 ** | −0.572 ** | −0.523 ** | −0.559 ** | −0.569 ** | −0.572 ** | |
| QL | Clay | −0.051 | 0.024 | −0.084 | −0.042 | 0.031 | −0.073 |
| Silt | 0.154 | 0.135 | 0.069 | 0.175 | 0.155 | 0.090 | |
| Sand | −0.058 | −0.100 | 0.019 | −0.076 | −0.117 | −0.002 | |
| FX | Clay | −0.027 | −0.034 | 0.073 | −0.017 | −0.023 | 0.085 |
| Silt | 0.589 ** | 0.570 ** | 0.558 ** | 0.586 ** | 0.572 ** | 0.575 ** | |
| Sand | −0.569 ** | −0.549 ** | −0.575 ** | −0.570 ** | −0.555 ** | −0.596 ** | |
| YC | Clay | 0.235 | −0.036 | −0.296 | 0.226 | −0.025 | −0.271 |
| Silt | 0.379 * | 0.344 | −0.131 | 0.410 * | 0.361 | −0.064 | |
| Sand | −0.366 * | −0.248 | 0.199 | −0.386 * | −0.264 | 0.140 | |
| AS | Clay | −0.023 | 0.012 | 0.068 | −0.025 | 0.008 | 0.056 |
| Silt | −0.132 | −0.161 | 0.184 | −0.146 | −0.179 | 0.137 | |
| Sand | 0.137 | 0.155 | −0.205 | 0.152 | 0.173 | −0.154 | |
| MZ | Clay | 0.022 | −0.240 | −0.159 | 0.029 | −0.215 | −0.142 |
| Silt | −0.157 | −0.218 | −0.313 | −0.144 | −0.193 | −0.287 | |
| Sand | 0.137 | 0.243 | 0.318 | 0.125 | 0.218 | 0.294 | |
| YL | Clay | −0.024 | −0.191 | −0.099 | −0.061 | −0.229 | −0.147 |
| Silt | 0.161 | −0.009 | 0.013 | 0.132 | −0.047 | −0.031 | |
| Sand | −0.101 | 0.079 | 0.029 | −0.067 | 0.120 | 0.077 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Z.; Chen, Z.; Xu, Y.; Ren, C.; Yang, G.; Han, X.; Ren, G.; Feng, Y. Relationship between Soil Organic Carbon Stocks and Clay Content under Different Climatic Conditions in Central China. Forests 2018, 9, 598. https://doi.org/10.3390/f9100598
Zhong Z, Chen Z, Xu Y, Ren C, Yang G, Han X, Ren G, Feng Y. Relationship between Soil Organic Carbon Stocks and Clay Content under Different Climatic Conditions in Central China. Forests. 2018; 9(10):598. https://doi.org/10.3390/f9100598
Chicago/Turabian StyleZhong, Zekun, Zhengxing Chen, Yadong Xu, Chengjie Ren, Gaihe Yang, Xinhui Han, Guangxin Ren, and Yongzhong Feng. 2018. "Relationship between Soil Organic Carbon Stocks and Clay Content under Different Climatic Conditions in Central China" Forests 9, no. 10: 598. https://doi.org/10.3390/f9100598
APA StyleZhong, Z., Chen, Z., Xu, Y., Ren, C., Yang, G., Han, X., Ren, G., & Feng, Y. (2018). Relationship between Soil Organic Carbon Stocks and Clay Content under Different Climatic Conditions in Central China. Forests, 9(10), 598. https://doi.org/10.3390/f9100598