Designer Niches Promote Seedling Survival in Forest Restoration: A 7-Year Study of Whitebark Pine (Pinus albicaulis) Seedlings in Waterton Lakes National Park
Abstract
:1. Introduction
2. Study Area and Methods
2.1. Study Area
2.2. Nursery Seedlings and Mycorrhizal Inoculation
2.3. Site Preparation
2.4. Planting Strategy and Treatments
2.5. Monitoring
2.6. Statistical Analysis
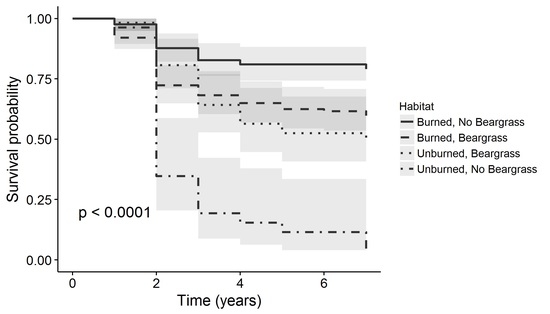
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grubb, P.J. The maintenance of species richness in plant communities: The importance of the regeneration niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Landres, P.B.; Morgan, P.; Swanson, F.J. Overview of the use of natural variability concepts in managing ecological systems. Ecol. Appl. 1999, 9, 1179–1188. [Google Scholar]
- Castro, J.; Allen, C.D.; Molina-Morales, M.; Jimènez, S.M.; Sànchez-Miranda, A.; Zamora, R. Salvage logging versus the use of burnt wood as a nurse object to promote post-fire tree seedling establishment. Restor. Ecol. 2011, 19, 537–544. [Google Scholar] [CrossRef]
- Maher, E.L.; Germino, M.J. Microsite differentiation among conifer species during seedling establishment at alpine treeline. Ecoscience 2006, 13, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Stanturf, J.A.; Palik, B.J.; Williams, M.I.; Karsten-Dumroese, R.; Madsen, P. Forest Restoration Paradigms. J. Sustain. For. 2014, 33 (Suppl. 1), S161–S194. [Google Scholar] [CrossRef] [Green Version]
- Arno, S.F.; Hoff, R.J. Pinus albicaulis Engelm. Whitebark pine. In Silvics of North America: Conifers; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; Volume 1, pp. 268–279. [Google Scholar]
- Tomback, D.F.; Achuff, P.; Schoettle, A.W.; Schwandt, J.; Mastrogiuseppe, R.J. The magnificent high-elevation five-needle white pines: Ecological roles and future outlook. In Proceedings High-Five Symposium: The Future of High-Elevation Five-Needle White Pines in Western North America; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2011; pp. 2–28. [Google Scholar]
- Tomback, D.F.; Linhart, Y.B. The evolution of bird-dispersed pines. Evolut. Ecol. 1990, 4, 185–219. [Google Scholar] [CrossRef]
- Tomback, D.F. Post-fire regeneration of krummolz whitebark pine: A consequence of nutcracker seed caching. Madrono 1986, 33, 100–110. [Google Scholar]
- Linhart, Y.B.; Tomback, D.F. Seed dispersal by nutcrackers causes multi-trunk growth form in pines. Oecologia 1985, 67, 107–110. [Google Scholar] [CrossRef] [PubMed]
- McCaughey, W.W.; Tomback, D.F. The Natural Regeneration Process; Island Press: Washington, DC, USA, 2001. [Google Scholar]
- Tomback, D.F.; Anderies, A.J.; Carsey, K.S.; Powell, M.L.; Mellmann-Brown, S. Delayed seed germination in whitebark pine and regeneration patterns following the Yellowstone fires. Ecology 2001, 82, 2587–2600. [Google Scholar] [CrossRef]
- Schwandt, J.W. Whitebark pine in peril: A Case for Restoration; US Department of Agriculture, Forest Service, Forest Health Protection: Missoula, MT, USA, 2006.
- Tomback, D.F.; Achuff, P. Blister rust and western forest biodiversity: ecology, values and outlook for white pines. For. Pathol. 2011, 40, 186–225. [Google Scholar] [CrossRef]
- Brar, S.; Tsui, C.K.; Dhillon, B.; Bergeron, M.J.; Joly, D.L.; Zambino, P.J.; Kassaby, Y.A.E.; Hamelin, R.C. Colonization history, host distribution, anthropogenic influence and landscape features shape populations of white pine blister rust, an invasive alien tree pathogen. PLoS ONE 2015, 10, e0127916. [Google Scholar] [CrossRef] [PubMed]
- Geils, B.W.; Vogler, D.R. A natural history of Cronartium ribicola. In Proceedings of the Future of High-Elevation, Five-Needle White Pines in Western North America: High Five Symposium, Fort Collins, CO, USA, 28–30 June 2010; pp. 210–217. [Google Scholar]
- Keane, R.E.; Tomback, D.F.; Aubry, C.A.; Bower, A.D.; Campbell, E.M.; Cripps, C.L.; Jenkins, M.B.; Mahalovich, M.F.; Manning, M.; McKinney, S.T.; et al. A Range-Wide Restoration Strategy for Whitebark Pine (Pinus albicaulis); US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2012.
- Tomback, D.F.; Arno, S.F.; Keane, R.E. The compelling case for management intervention. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 3–25. [Google Scholar]
- Committee on the Status of Endangered Wildlife in Canada. COSEWIC Assessment and Status Report on the Whitebark Pine (Pinus albicaulis) in Canada-2010; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2010.
- Alberta Whitebark Pine Recovery Team. Alberta Whitebark Pine Recovery Plan 2013–2018; Alberta Recovery Plan No. 34; Alberta Environment and Sustainable Resource Development, Alberta Whitebark Pine Recovery Team: Edmonton, AB, Canada, 2014; p. 63. [Google Scholar]
- Environment and Climate Change Canada. Recovery Strategy for the Whitebark Pine (Pinus albicaulis) in Canada [Proposed]; Environment and Climate Change Canada: Ottawa, ON, Canada, 2017.
- Larson, E.R.; Kipfmueller, K.F. Patterns in whitebark pine regeneration and their relationships to biophysical site characteristics in southwest Montana, central Idaho, and Oregon, USA. Can. J. For. Res. 2010, 40, 476–487. [Google Scholar] [CrossRef]
- Tomback, D.F.; Sund, S.K.; Hoffman, L.A. Postfire regeneration of Pinus albicaulis: Height—age relationships, age structure, and microsite characteristics. Can. J. For. Res. 1993, 23, 113–119. [Google Scholar] [CrossRef]
- Gelderman, M.S.; Macdonald, S.E.; Gould, A.J. Regeneration niche of whitebark pine in the Canadian Rocky Mountains: The basis to restoring an endangered species. Arct. Antarct. Alpine Res. 2016, 48, 279–292. [Google Scholar] [CrossRef]
- McCaughey, W.; Scott, G.L.; Izlar, K.L. Whitebark Pine Planting Guidelines. West. J. Appl. For. 2009, 24, 163–166. [Google Scholar]
- Izlar, D.K. Assessment of Whitebark Pine Seedling Survival for Rocky Mountain Plantings. Master’s Thesis, University of Montana, Missoula, MT, USA, 2007. [Google Scholar]
- Lonergan, E.R.; Cripps, C.L.; Smith, C.M. Influence of site conditions, shelter objects, and ectomycorrhizal inoculation on the early Survival of whitebark pine seedlings planted in Waterton Lakes National Park. For. Sci. 2014, 60, 603–612. [Google Scholar] [CrossRef]
- Cripps, C.L.; Longeran, E.R.; Smith, C. Survival of whitebark seedlings inoculated with ectomycorrhiza. Nutcracker Notes 2014, 26, 15–18. [Google Scholar]
- Perkins, J.L. Fire enhances whitebark pine seedling establishment, survival, and growth. Fire Ecol. 2015, 11, 84–99. [Google Scholar] [CrossRef]
- Mellmann-Brown, S. Regeneration of whitebark pine in the timberline ecotone of the Beartooth Plateau, USA: spatial distribution and responsible agents. In Mountain Ecosystems: Studies in Treeline Ecology; Broll, G., Keplin, B., Eds.; Springer: Berlin, Germany, 2005; pp. 97–115. [Google Scholar]
- Mahalovich, M.F.; Burr, K.E.; Foushee, D.L. Whitebark pine germination, rust resistance, and cold hardiness among seed sources in the Inland Northwest: Planting strategies for restoration. In Proceedings of the Forest and Conservation Nursery Associations; Proc. RMRS-P-43; Riley, L.E., Dumroese, R.K., Landis, T.D., tech coords, Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006. [Google Scholar]
- Smith, C.M.; Wilson, B.; Rasheed, S.; Walker, R.C.; Carolin, T.; Shepherd, B. Whitebark pine and white pine blister rust in the Rocky Mountains of Canada and northern Montana. Can. J. For. Res. 2008, 38, 982–995. [Google Scholar] [CrossRef]
- Smith, C.M.; Shepherd, B.; Gillies, C.; Stuart-Smith, J. Changes in blister rust infection and mortality in whitebark pine over time. Can. J. For. Res. 2012, 43, 90–96. [Google Scholar] [CrossRef]
- Shepherd, B.; Jones, B.; Sissons, R.; Cochrane, J.; Park, J.; Smith, C.M.; Stafl, N. Ten years of monitoring illustrates a cascade of effects of white pine blister rust and focuses whitebark pine restoration in the Canadian Rocky and Columbia Mountains. Forests 2018, 9, 138. [Google Scholar] [CrossRef]
- Achuff, P.; McNeil, R.; Coleman, M.L.; Wallis, C.; Wershler, C. Ecological Land Classification of Waterton Lakes National Park, Alberta; Canadian Forest Service: Waterton Park, AL, Canada, 2002; p. 226.
- Burr, K.E.; Eramian, A.; Eggleston, K. Growing whitebark pine seedlings for restoration. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 325–345. [Google Scholar]
- Lonergan, E.R.; Cripps, C.L. Use of low nitrogen fertilizer as a strategy for maintaining mycorrhizal colonization on whitebark pine seedlings inoculated with native fungi in the greenhouse. Native Plants J. 2013, 14, 213–224. [Google Scholar] [CrossRef]
- Schwanke, R.; Smith, C.M. Imitating lightning strikes for whitebark pine restoration. Nutcracker Notes 2010, 19, 12–13. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Goel, M.; Khanna, P.; Kishore, J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274. [Google Scholar] [Green Version]
- Asebrook, J.M.; Lapp, J.; Carolin, T. Whitebark and limber pine restoration and monitoring in Glacier National Park. In Proceedings of the Future of High-Elevation, Five-Needle White Pines in Western North America: High Five Symposium, Fort Collins, CO, USA, 28–30 June 2010. [Google Scholar]
- Moody, R.J. Post-Fire Regeneration and Survival of Whitebark Pine (Pinus albicaulis Engelm.). Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2006. [Google Scholar]
- Keane, R.E.; Parsons, R. Restoring whitebark pine forests of the Northern Rocky Mountains, USA. Ecol. Restor. 2010, 28, 56–70. [Google Scholar] [CrossRef]
- Harvey, B.J.; Donato, D.C.; Turner, M.G. High and dry: post-fire tree seedling establishment in subalpine forests decreases with post-fire drought and large stand-replacing burn patches. Glob. Ecol. Biogeogr. 2016, 25, 655–669. [Google Scholar] [CrossRef] [Green Version]
- Barringer, L.E.; Tomback, D.F.; Wunder, M.B.; McKinney, S.T. Whitebark pine stand condition, tree abundance, and cone production as predictors of visitation by Clark’s nutcracker. PLoS ONE 2012, 7, e37663. [Google Scholar] [CrossRef] [PubMed]
- Leirfallom, S.B.; Keane, R.E.; Tomback, D.F.; Dobrowski, S.Z. The effects of seed source health on whitebark pine (Pinus albicaulis) regeneration density after wildfire. Can. J. For. Res. 2015, 45, 1597–1606. [Google Scholar] [CrossRef]
- Klutsch, J.G.; Goodrich, B.A.; and Jacobi, W.R. Post-fire regeneration dynamics in whitebark pine (Pinus albicaulis) forests in wind river and Absaroka Mountains, Wyoming, USA. J. For. Res. 2015, 26, 719–733. [Google Scholar] [CrossRef]
- Goeking, S.; Izlar, D.K. Pinus albicaulis Engelm. (whitebark pine) in mixed-species stands throughout its US range: Broad-scale indicators of extent and recent decline. Forests 2018, 9, 131. [Google Scholar] [CrossRef]
- Kohout, P.; Sykorova, Z.; Bahram, M.; Hadincova, V.; Albrechtova, J.; Tedersoo, L.; Vohnik, M. Ericaceous dwarf shrubs affect ectomycorrhizal fungal community of the invasive Pinus strobus and native Pinus sylvestris in a pot experiment. Mycorrhiza 2011, 21, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Wiensczyk, G.; Durall, D.; Jones, M.; Simard, S. Ectomycorrhizae and forestry in British Columbia: A summary of current research and conservation strategies. J. Ecosyst. Manag. 2002, 2, 1–20. [Google Scholar]
- Kropp, B.R.; Langlois, C.G. Ectomycorrhizal fungi in restoration. Can. J. For. Res. 1990, 20, 43–8451. [Google Scholar]
- Jenkins, M.; Cripps, C.L.; Germaine, L.E. Scorched earth: Suillus colonization of Pinus albicaulis seedlings planted in wildfire-impacted soil affects seedling biomass, foliar nutrient content, and isotope signatures. Plant Soil 2018, 425, 113–131. [Google Scholar] [CrossRef]
- Antibus, R.; Hobbie, E.A.; Cripps, C.L. Sporocarp δ15 N and use of inorganic and organic nitrogen in vitro differ among host-specific suilloid fungi associated with high elevation five-needle pines. Mycoscience 2018, in press. [Google Scholar] [CrossRef]
- Trusty, P.; Cripps, C.L. Influence of fire on mycorrhizal colonization of planted and natural whitebark pine seedlings: Ecology and management implications. In Proceedings High-Five Symposium: The Future of High-Elevation Five-Needle White Pines in Western North America; Keane, R.E., Tomback, D.F., Murray, M.P., Smith, C.M., Eds.; Rocky Mountain Research Station: Fort Collins, CO, USA, 2011; pp. 198–202. [Google Scholar]
- Mohatt, K.R.; Cripps, C.L.; Lavin, M. Ectomycorrhizal fungi of whitebark pine (a tree in peril) revealed by sporocarps and molecular analysis of mycorrhizae from treeline forests in the Greater Yellowstone Ecosystem. Botany 2008, 86, 14–25. [Google Scholar] [CrossRef]
- Cripps, C.L.; Antibus, R.K. Native ectomycorrhizal fungi of limber and whitebark pine: Necessary for forest sustainability. In Proceedings of the Future of High-Elevation, Five-Needle White Pines in Western North America: High Five Symposium, Fort Collins, CO, USA, 28–30 June 2010. [Google Scholar]
- Treu, R.; Karst, J.; Randall, M.; Pec, G.J.; Cigan, P.; Simard, S.W.; Cooke, J.E.K. Decline of ectomycorrhizal fungi following a mountain pine beetle epidemic. Ecology 2014, 95, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Rainer, G.; Kuhnert, R.; Unterholzer, M.; Dresch, P.; Gruber, A.; Peintner, U. Host-specialist dominated ectomycorrhizal communities of Pinus cembra are not affected by temperature manipulation. J. Fungi 2015, 1, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Keane, R.E.; Holsinger, L.M.; Mahalovich, M.F.; Tomback, D.F. Evaluating future success of whitebark pine ecosystem restoration under climate change using simulation modeling. Restor. Ecol. 2017, 25, 220–233. [Google Scholar] [CrossRef]
- Keane, R.E.; Holsinger, L.M.; Mahalovich, M.F.; Tomback, D.F. Restoring Whitebark Pine Ecosystems in the Face of Climate Change; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2017.
- Landguth, E.L.; Holden, Z.A.; Mahalovich, M.F.; Cushman, S.A. Using landscape genetics simulations for planting blister rust resistant whitebark pine in the US northern Rocky Mountains. Front. Genet. 2017, 8, 1–9. Available online: https://doi.org/10.3389/fgene.2017.00009 (accessed on 2 July 2018). [CrossRef] [PubMed]





| Seedling Individuals Seedling Clusters | |||||
|---|---|---|---|---|---|
| Treatment | N | Survival | n | Survival | |
| Overall | 990 | 53.0 | 331 | 60.8 | |
| Habitat | Burn, No Beargrass | 366 | 67.3 | 122 | 79.0 |
| Burn, Beargrass | 374 | 53.3 | 125 | 59.7 | |
| No Burn, Beargrass | 169 | 39.9 | 57 | 50.3 | |
| No Burn, No Beargrass | 81 | 12.9 | 27 | 3.9 | |
| Microsite | Present | 658 | 62.1 | 219 | 70.7 |
| Absent | 332 | 35.1 | 112 | 41.5 | |
| Beargrass | Present | 543 | 49.2 | 182 | 56.9 |
| Absent | 447 | 57.6 | 149 | 65.5 | |
| Burn | Present | 740 | 60.3 | 247 | 69.3 |
| Absent | 250 | 31.2 | 84 | 35.1 | |
| Microsite Type | Stump | 74 | 67.1 | 25 | 79.6 |
| Snag | 231 | 71.9 | 77 | 76.9 | |
| Rock | 78 | 54.4 | 26 | 69.2 | |
| Log | 275 | 54.8 | 91 | 63.2 | |
| None | 332 | 35.1 | 112 | 41.5 | |
| Inoculation and Burn | Exposed, Burn | 552 | 59.2 | 182 | 69.0 |
| Un-inoculated, Burn | 188 | 63.5 | 65 | 70.3 | |
| Exposed, No Burn | 169 | 33.5 | 57 | 35.8 | |
| Un-inoculated, No Burn | 81 | 25.8 | 27 | 33.3 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cripps, C.L.; Alger, G.; Sissons, R. Designer Niches Promote Seedling Survival in Forest Restoration: A 7-Year Study of Whitebark Pine (Pinus albicaulis) Seedlings in Waterton Lakes National Park. Forests 2018, 9, 477. https://doi.org/10.3390/f9080477
Cripps CL, Alger G, Sissons R. Designer Niches Promote Seedling Survival in Forest Restoration: A 7-Year Study of Whitebark Pine (Pinus albicaulis) Seedlings in Waterton Lakes National Park. Forests. 2018; 9(8):477. https://doi.org/10.3390/f9080477
Chicago/Turabian StyleCripps, Cathy L., Genoa Alger, and Robert Sissons. 2018. "Designer Niches Promote Seedling Survival in Forest Restoration: A 7-Year Study of Whitebark Pine (Pinus albicaulis) Seedlings in Waterton Lakes National Park" Forests 9, no. 8: 477. https://doi.org/10.3390/f9080477
APA StyleCripps, C. L., Alger, G., & Sissons, R. (2018). Designer Niches Promote Seedling Survival in Forest Restoration: A 7-Year Study of Whitebark Pine (Pinus albicaulis) Seedlings in Waterton Lakes National Park. Forests, 9(8), 477. https://doi.org/10.3390/f9080477