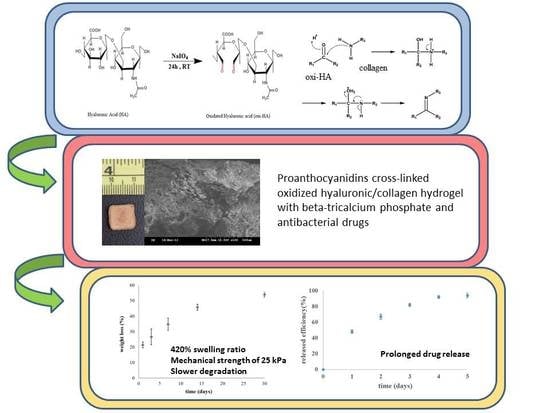
Integrated Oxidized-Hyaluronic Acid/Collagen Hydrogel with β-TCP Using Proanthocyanidins as a Crosslinker for Drug Delivery
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation and Characterizations of CH Polymers
2.2.1. Oxidized HA (oxi-HA)
2.2.2. CH Polymers
2.2.3. Pretreatment of Collagen Samples
2.2.4. Crosslinking Index
2.2.5. Preparation of Hydrogel
2.2.6. Characterizations Using Fourier Transform Infrared Spectrometer (FTIR) and Scanning Electron Microscope (SEM)
2.3. Preparation of Composite Materials, CHT and CHTP
2.4. Degradation and Swelling Properties of Composite Material
2.5. Evaluation of Compression Testing Using a Universal Testing Machine (BOSE, USA)
2.6. Biocompatibility Investigation
2.6.1. Cell Cultures
2.6.2. MTT Viability Assay
2.7. Drug Loading and Drug Release
2.8. Statistical Analysis
3. Results and Discussions
3.1. CH Hydrogel
3.1.1. Characterization of Oxidized Hyaluronic Acid/Collagen Hydrogels (CH)
3.1.2. SEM Morphology of CH Hydrogel
3.1.3. Cross-Linking Index
3.2. CHT Composite Material
3.2.1. Swelling and Mechanical Test
3.2.2. Degradation Test
3.3. CHTP Composite Material
3.3.1. Degradation Test
3.3.2. Characterizations of CHTP Composite Material
3.3.3. Drug Releasing Profile
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2014, 64, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J.; Grossman, N.S. Periodontal Disease as a Specific, albeit Chronic, Infection: Diagnosis and Treatment. Clin. Microbiol. Rev. 2001, 14, 727–752. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Bhavsar, C.; Sawarkar, S.; D’souza, A. Current and novel approaches for control of dental biofilm. Int. J. Pharm. 2018, 536, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Meimandi, M.; Talebi Ardakani, M.R.; Esmaeil Nejad, A.; Yousefnejad, P.; Saebi, K.; Tayeed, M.H. The Effect of Photodynamic Therapy in the Treatment of Chronic Periodontitis: A Review of Literature. J. Lasers Med. Sci. 2017, 8, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Andrei, M.; Dinischiotu, A.; Didilescu, A.C.; Ionita, D.; Demetrescu, I. Periodontal materials and cell biology for guided tissue and bone regeneration. Ann. Anat. Anat. Anz. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Kim, S.-G. Membranes for the Guided Bone Regeneration. Maxillofac. Plast. Reconstr. Surg. 2014, 36, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, T.; Omata, K.; Hashimoto, Y.; Tabata, Y.; Satoh, T. Alveolar bone tissue engineering using composite scaffolds for drug delivery. Jpn. Dent. Sci. Rev. 2010, 46, 188–192. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Helling, A.L.; Tsekoura, E.K.; Biggs, M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. In Vitro Enzymatic Degradation of Tissue Grafts and Collagen Biomaterials by Matrix Metalloproteinases: Improving the Collagenase Assay. ACS Biomater. Sci. Eng. 2017, 3, 1922–1932. [Google Scholar] [CrossRef]
- Hatem, A.; Malle, E.; Koyani, C.N.; Pussinen, P.J.; Sorsa, T. Neutrophil proteolytic activation cascades: A possible mechanistic link between chronic periodontitis and coronary heart disease. Innate Immun. 2015, 22, 85–99. [Google Scholar]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Ma, B.; Wang, X.; Wu, C.; Chang, J. Crosslinking strategies for preparation of extracellular matrix-derived cardiovascular scaffolds. Regen. Biomater. 2014, 1, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Yao, J.; Shao, Z.; Chen, X. Strong Collagen Hydrogels by Oxidized Dextran Modification. ACS Sustain. Chem. Eng. 2014, 2, 1318–1324. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Maietta, S.; Russo, T.; Santis, R.; Ronca, D.; Riccardi, F.; Catauro, M.; Martorelli, M.; Gloria, A. Further Theoretical Insight into the Mechanical Properties of Polycaprolactone Loaded with Organic–Inorganic Hybrid Fillers. Materials 2018, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, N.; Felice, P.; Pellegrino, G.; Camurati, A.; Gambino, P.; Esposito, M. Guided bone regeneration with and without a bone substitute at single post-extractive implants: 1-year post-loading results from a pragmatic multicentre randomised controlled trial. Eur. J. Oral Implantol. 2011, 4, 313–325. [Google Scholar] [PubMed]
- Wang, T.; Zhang, G.; Zhou, G.; Yu, X.; Zhang, J.; Wang, X.; Tang, Z. Comparative evaluation of a biomimic collagen/hydroxyapatite/β-tricaleium phosphate scaffold in alveolar ridge preservation with Bio-Oss Collagen. Front. Mater. Sci. 2016, 10, 122–133. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Lo, Y.J.; Feng, S.W.; Huang, Y.C.; Tsai, H.Y.; Lin, C.T.; Fan, K.H.; Huang, H.M. Bone Healing Improvements Using Hyaluronic Acid and Hydroxyapatite/Beta-Tricalcium Phosphate in Combination: An Animal Study. BioMed Res. Int. 2016, 2016, 8301624. [Google Scholar] [CrossRef] [PubMed]
- Segari, W.A.O.; El Khalek Radwan, D.A.; Abd El Hamid, M.A. The effect of adding hyaluronic acid to calcium phosphate on periapical tissue healing following periradicular surgery in dogs. Tanta Dent. J. 2014, 11, 122–129. [Google Scholar] [CrossRef]
- Motamedian, S.R.; Hosseinpour, S.; Ahsaie, M.G.; Khojasteh, A. Smart scaffolds in bone tissue engineering: A systematic review of literature. World J. Stem Cells 2015, 7, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Albani, D.; Gloria, A.; Tunesi, M.; Batelli, S.; Russo, T.; Forloni, G.; Ambrosio, L.; Cigada, A. Multidisciplinary Perspectives for Alzheimer’s and Parkinson’s Diseases: Hydrogels for Protein Delivery and Cell-Based Drug Delivery as Therapeutic Strategies. Int. J. Artif. Org. 2009, 32, 836–850. [Google Scholar] [CrossRef]
- Ma, S.; Adayi, A.; Liu, Z.; Li, M.; Wu, M.; Xiao, L.; Sun, Y.; Cai, Q.; Yang, X.; Zhang, X.; et al. Asymmetric Collagen/chitosan Membrane Containing Minocycline-loaded Chitosan Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2016, 6, 31822. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Kumar, S.; Dagli, N.; Dagli, R.J. Effect of tetracycline HCl in the treatment of chronic periodontitis—A clinical study. J. Int. Soc. Prev. Community Dent. 2014, 4, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Mahendra, J.; Ari, G. Minocycline Ointment as a Local Drug Delivery in the Treatment of Generalized Chronic Periodontitis—A Clinical Study. J. Clin. Diagn. Res. 2016, 10, ZC15–ZC19. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Kokol, V. Collagen- Vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives, Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTech: London, UK, 2011; Available online: https://www.intechopen.com/books/biomaterials-applications-for-nanomedicine/collagen-vs-gelatine-based-biomaterials-and-their-biocompatibility-review-and-perspectives (accessed on 19 October 2017). [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Guo, X.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Kasper, F.K.; Mikos, A.G. Effect of Swelling Ratio of Injectable Hydrogel Composites on Chondrogenic Differentiation of Encapsulated Rabbit Marrow Mesenchymal Stem Cells In Vitro. Biomacromolecules 2009, 10, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.V.; Shivakumar, H.G. Investigation of Swelling Behavior and Mechanical Properties of a pH-Sensitive Superporous Hydrogel Composite. Iran. J. Pharm. Res. 2012, 11, 481–493. [Google Scholar] [PubMed]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Meyvis, T.K.L.; De Smedt, S.C.; Demeester, J.; Hennink, W.E. Influence of the Degradation Mechanism of Hydrogels on Their Elastic and Swelling Properties during Degradation. Macromolecules 2000, 33, 4717–4725. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.Y.; Chen, W.S.; Sun, J.S.; Lin, F.H.; Wu, T. Biological characterization of oxidized hyaluronic acid/resveratrol hydrogel for cartilage tissue engineering. J. Biomed. Mater. Res. A 2013, 101, 3457–3466. [Google Scholar] [CrossRef] [PubMed]
- De Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-Y.; Chen, Y.-C.; Lin, F.-H. Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration. Acta Biomater. 2010, 6, 3044–3055. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.D.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Killion, J.A.; Geever, L.M.; Devine, D.M.; Farrell, H.; Higginbotham, C.L. Compressive Strength and Bioactivity Properties of Photopolymerizable Hybrid Composite Hydrogels for Bone Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 641–650. [Google Scholar] [CrossRef]
- Wu, J.; He, J.Y.; Odegard, G.M.; Zhang, Z.L. Effect of chain architecture on the compression behavior of nanoscale polyethylene particles. Nanoscale Res. Lett. 2013, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Ou, R.; Zhang, H.C.; Kim, S.; Simon, G.P.; Hou, H.J.; Wang, H.T. Improvement of the Swelling Properties of Ionic Hydrogels by the Incorporation of Hydrophobic, Elastic Microfibers for Forward Osmosis Applications. Ind. Eng. Chem. Res. 2017, 56, 505–512. [Google Scholar] [CrossRef]
- Okay, O. General Properties of Hydrogels, in Hydrogel Sensors and Actuators: Engineering and Technology; Gerlach, G., Arndt, K.-F., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–14. [Google Scholar]
- Hechler, B.; Yao, X.; Wang, Y. Proanthocyanidins Alter Adhesive/Dentin Bonding Strengths when Included in a Bonding System. Am. J. Dent. 2012, 25, 276–280. [Google Scholar] [PubMed]
- Green, B.; Yao, X.M.; Ganguly, A.; Xu, C.Q.; Dusevich, V.; Walker, M.P.; Wang, Y. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. J. Dent. 2010, 38, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y. Effect of proanthocyanidins and photo-initiators on photo-polymerization of a dental adhesive. J. Dent. 2013, 41, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-R.; Fang, M.; Zhang, L.; Tang, C.-F.; Dou, Q.; Chen, J.-H. Anti-proteolytic capacity and bonding durability of proanthocyanidin-biomodified demineralized dentin matrix. Int. J. Oral Sci. 2014, 6, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Tian, L.L.; Ramakrishna, S.; Dehghani, L. Structural properties of scaffolds: Crucial parameters towards stem cells differentiation. World J. Stem Cells 2015, 7, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bartolo, P. Enhancing the Hydrophilicity and Cell Attachment of 3D Printed PCL/Graphene Scaffolds for Bone Tissue Engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.G.; Mohanty, S.; Ray, A.R.; Malhotra, R.; Aran, B. Culture & differentiation of mesenchymal stem cell into osteoblast on degradable biomedical composite scaffold: In vitro study. Ind. J. Med. Res. 2015, 142, 747–758. [Google Scholar]
- Fine, A.M. Oligomeric proanthocyanidin complexes: History, structure, and phytopharmaceutical applications. Altern Med. Rev. 2000, 5, 144–151. [Google Scholar] [PubMed]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Ray, S.D.; Sen, C.K.; Preuss, H.G. Cellular Protection with Proanthocyanidins Derived from Grape Seeds. Ann. N. Y. Acad. Sci. 2002, 957, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, L.G.; Zhao, Z.Q.; Shi, J.; Wang, X.; Huang, J. Grape seed proanthocyanidins inhibit H2O2-induced osteoblastic MC3T3-E1 cell apoptosis via ameliorating H2O2-induced mitochondrial dysfunction. J. Toxicol. Sci. 2014, 39, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Park, M.-K.; Oh, H.-J.; Woo, Y.-J.; Lim, M.-A.; Lee, J.-H.; Ju, J.H.; Jung, Y.O.; Lee, Z.H.; Park, S.-H.; et al. Grape-Seed Proanthocyanidin Extract as Suppressors of Bone Destruction in Inflammatory Autoimmune Arthritis. PLoS ONE 2012, 7, e51377. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Fraimow, H.S. Systemic Antimicrobial Therapy in Osteomyelitis. Semin. Plast. Surg. 2009, 23, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Soares, G.M.S.; Figueiredo, L.C.; Faveri, M.; Cortelli, S.C.; Duarte, P.M.; Feres, M. Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. J. Appl. Oral Sci. 2012, 20, 295–304. [Google Scholar] [CrossRef] [PubMed]








| Polymer | Components | Abbreviation |
|---|---|---|
| A | Oxidized hyaluronic acid | (oxi-HA) |
| B | A + Collagen | CH |
| C | B + β-tricalcium phosphate (β-TCP) | CHT |
| D | B + β-TCP + OPCs | CHTP |
| Name | Concentrations in g/L or mL/L |
|---|---|
| Minimum essential medium (MEM) | 9.3918 g |
| Non-essential amino acid (NEAA) | 10 mL |
| Sodium pyruvate (SP) | 10 mL |
| L-glutamine (L-G) | 10 mL |
| Prostate-specific antigen (PSA) | 10 mL |
| Sodium bicarbonate | 1.5 g |
| Heat-inactivated fetal bovine serum | 100 mL |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Chang, Y.-H.; Liu, C.-J.; Chung, R.-J. Integrated Oxidized-Hyaluronic Acid/Collagen Hydrogel with β-TCP Using Proanthocyanidins as a Crosslinker for Drug Delivery. Pharmaceutics 2018, 10, 37. https://doi.org/10.3390/pharmaceutics10020037
Wei Y, Chang Y-H, Liu C-J, Chung R-J. Integrated Oxidized-Hyaluronic Acid/Collagen Hydrogel with β-TCP Using Proanthocyanidins as a Crosslinker for Drug Delivery. Pharmaceutics. 2018; 10(2):37. https://doi.org/10.3390/pharmaceutics10020037
Chicago/Turabian StyleWei, Yang, Yu-Han Chang, Chung-Jui Liu, and Ren-Jei Chung. 2018. "Integrated Oxidized-Hyaluronic Acid/Collagen Hydrogel with β-TCP Using Proanthocyanidins as a Crosslinker for Drug Delivery" Pharmaceutics 10, no. 2: 37. https://doi.org/10.3390/pharmaceutics10020037
APA StyleWei, Y., Chang, Y. -H., Liu, C. -J., & Chung, R. -J. (2018). Integrated Oxidized-Hyaluronic Acid/Collagen Hydrogel with β-TCP Using Proanthocyanidins as a Crosslinker for Drug Delivery. Pharmaceutics, 10(2), 37. https://doi.org/10.3390/pharmaceutics10020037