Material Considerations for Fused-Filament Fabrication of Solid Dosage Forms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Filaments by HME
2.3. Production Tablets by FFF
2.4. Mechanical Testing
2.4.1. Dynamic Mechanical Analysis
2.4.2. Filament Stiffness
2.4.3. Filament Brittleness
2.5. Melt Flow Indexing
2.6. Differential Scanning Calorimetry
2.7. Mass Loss Studies
2.8. Direct Compression
2.9. Drug Release Studies
2.10. Scanning Electron Microscopy
2.11. Statistical Analysis
3. Results
3.1. Mechanical Characterization
3.1.1. Filament Stiffness
3.1.2. Filament Brittleness
3.1.3. Dynamic Mechanical Analysis
3.2. Thermal Characterization
3.3. Dissolution Studies
3.3.1. Mass Loss
3.3.2. Cumulative Drug Release
4. Discussion
4.1. Material Formulation Rationale
4.2. Filament Production
4.3. Filament Characterization
4.4. 3D Printing of Flat-Faced Tablets
4.5. Tablet Properties
4.6. Material Considerations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. An evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, G.; Karim, A. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Yoo, J.; Thomas, J.; Bradbury; Bebb, T.J.; Iskra, J.; Surprenant, H.L.; West, T.G. Three-Dimensional Printing System and Equipment Assembly. U.S. Patent 9,517,591, 13 December 2016. [Google Scholar]
- Tiwari, R.V.; Patil, H.; Repka, M. A Contribution of hot-melt extrusion technology to advance drug delivery in the 21st century. Expert Opin. Drug Deliv. 2016, 13, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Gholizadeh, H.; Lu, J.; Bunt, C.; Seyfoddin, A. Application of Fused Deposition Modelling (FDM) Method of 3D Printing in Drug Delivery. Curr. Pharm. Des. 2017, 23, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Fukushige, K.; Ogawa, E.; Hayashi, N.; Ozeki, T. 3D Printing Factors Important for the Fabrication of Polyvinylalcohol Filament-Based Tablets. Biol. Pharm. Bull. 2017, 40, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.M.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci. Rep. 2017, 7, 2829. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Chang, H.; Sedough, D.; Hatton, G.B.; Wang, J.; Buanz, A.; Gaisford, S.; Basit, A.W. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int. J. Pharm. 2015, 496, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.C.R.; Chaves, P.S.; Goyanez, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.W.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.C.; Pereira, B.C.; Arafat, B.; Cieszynska, M.; Isreb, A.; Alhnan, M.A. Fabricating a Shell-Core Delayed Release Tablet Using Dual FDM 3D Printing for Patient-Centred Therapy. Pharm. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.W.; Alhnan, M.A. A Lower Temperature FDM 3D Printing for the Manufacture of Patient-Specific Immediate Release Tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Gioumouxouzis, C.I.; Katsamenis, O.L.; Bouropoulos, N.; Fatouros, D.G. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 164–171. [Google Scholar] [CrossRef]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Kobayashi, M.; Martínez-Pacheco, R.; Gaisford, S.; Basit, A.W. Fused-filament 3D printing of drug products: Microstructure analysis and drug release characteristics of PVA-based caplets. Int. J. Pharm. 2016, 514, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [CrossRef] [PubMed]
- Holländer, J.; Genina, N.; Jukarainen, H.; Khajeheian, M.; Rosling, A.; Mäkilä, E.; Sandler, N. Three-Dimensional Printed PCL-Based Implantable Prototypes of Medical Devices for Controlled Drug Delivery. J. Pharm. Sci. 2016, 105, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Genina, N.; Holländer, J.; Jukarainen, H.; Mäkilä, E.; Salonen, J.; Sandler, N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur. J. Pharm. Sci. 2016, 90, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Kempin, W.; Franz, C.; Koster, L.; Schneider, F.; Bogdahn, M.; Weitschies, W.; Seidlitz, A. Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. Eur. J. Pharm. Biopharm. 2017, 115, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Weisman, J.A.; Nicholson, J.C.; Tappa, K.; Jammalamadaka, U.; Wilson, C.G.; Mills, D.K. Antibiotic and chemotherapeutic enhanced three-dimensional printer filaments and constructs for biomedical applications. Int. J. Nanomed. 2015, 357. [Google Scholar] [CrossRef]
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Debotton, N.; Dahan, A. Applications of Polymers as Pharmaceutical Excipients in Solid Oral Dosage Forms. Med. Res. Rev. 2017, 37, 52–97. [Google Scholar] [CrossRef] [PubMed]
- Major, I.; Fuenmayor, E.; McConville, C. The Production of Solid Dosage Forms from Non-Degradable Polymers. Curr. Pharm. Des. 2016, 22, 2738–2760. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.P.; Kuentz, M.; Holm, R. Pharmaceutical excipients-quality, Regulatory and biopharmaceutical considerations. Eur. J. Pharm. Sci. 2016, 87, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Evans, J. 3D printing makes material advances: Companies are stepping up their efforts to expand the limited palette of resins suitable for additive manufacturing. Plast. Eng. 2016, 72, 32–34. [Google Scholar]
- Gilmer, E.L.; Miller, D.; Chatham, C.A.; Zawaski, C.; Fallon, J.J.; Pekkanen, A.; Long, T.E.; Williams, C.B.; Bortner, M.J. Model analysis of feedstock behavior in fused filament fabrication: Enabling rapid materials screening. Polymer 2017. [Google Scholar] [CrossRef]
- Bühler, V. Kollidon, V. Kollidon® Polyvinylpyrrolidone excipients. Pharm. Ind. 2008, 9, 207–220. [Google Scholar]
- Forde, M.; Lyons, J.G.; Devine, D.M.; McConville, C.; Major, I. Dual-layer Vaginal Tablet for the Treatment of Cervical Cancer. In Proceedings of the Irish Association for Cancer Research Annual Meeting 2017, Kilkenny, Ireland, 23–24 February 2017. [Google Scholar]
- McConville, C.; Major, I.; Devlin, B.; Brimer, A. Development of a multi-layered vaginal tablet containing dapivirine, levonorgestrel and acyclovir for use as a multipurpose prevention technology. Eur. J. Pharm. Biopharm. 2016, 104, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Brostow, W.; Hagg Lobland, H.E.; Narkis, M. Sliding wear, viscoelasticity, and brittleness of polymers. J. Mater. Res. 2006, 21, 2422–2428. [Google Scholar] [CrossRef]
- Lyons, J.G.; Blackie, P.; Higginbotham, C.L. The significance of variation in extrusion speeds and temperatures on a PEO/PCL blend based matrix for oral drug delivery. Int. J. Pharm. 2008, 351, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Verreck, G.; Decorte, A.; Li, H.; Tomasko, D.; Arien, A.; Peeters, J.; Rombaut, P.; Van den Mooter, G.; Brewster, M.E. The effect of pressurized carbon dioxide as a plasticizer and foaming agent on the hot melt extrusion process and extrudate properties of pharmaceutical polymers. J. Supercrit. Fluids 2006, 38, 383–391. [Google Scholar] [CrossRef]
- Bley, H.; Fussnegger, B.; Bodmeier, R. Characterization and stability of solid dispersions based on PEG/polymer blends. Int. J. Pharm. 2010, 390, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Thiry, J.; Lebrun, P.; Vinassa, C.; Adam, M.; Netchacovitch, L.; Ziemons, E.; Hubert, P.; Krier, F.; Evrard, B. Continuous production of itraconazole-based solid dispersions by hot melt extrusion: Preformulation, optimization and design space determination. Int. J. Pharm. 2016, 515, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, L.; Yang, P.; Wenslow, R.M.; Tan, B.; Zhang, H.; Deng, Z. Physicochemical Characterization of Felodipine-Kollidon VA64 Amorphous Solid Dispersions Prepared by Hot-Melt Extrusion. J. Pharm. Sci. 2013, 102, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Lehmkemper, K.; Kyeremateng, S.O.; Degenhardt, M.; Sadowski, G. Influence of Low-Molecular-Weight Excipients on the Phase Behavior of PVPVA64 Amorphous Solid Dispersions. Pharm. Res. 2018, 35. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, F.; Zhang, C.; Ping, Q. Use of polymer combinations in the preparation of solid dispersions of a thermally unstable drug by hot-melt extrusion. Acta Pharm. Sin. B 2013, 3, 263–272. [Google Scholar] [CrossRef]
- Li, Y.; Lu, M.; Wu, C. PVP VA64 as a novel release-modifier for sustained-release mini-matrices prepared via hot melt extrusion. Drug Deliv. Transl. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.M.; Le, K.H.T.; Giannitelli, S.M.; Lee, Y.J.; Rainer, A.; Trombetta, M. Electrospinning of PCL/PVP blends for tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2013, 24, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Elosegui-Artola, A.; Le, K.H.T.; Kim, G.M. Morphological cues for regulation of cell adhesion and motility with tailored electrospun scaffolds of pcl and pcl/pvp blends. Cell. Mol. Bioeng. 2013, 6, 482–492. [Google Scholar] [CrossRef]
- Shonaike, G.O.; Simon, G.P. Polymer Blends and Alloys; Marcel Dekker: New York, NY, USA, 1999; ISBN 0824719808. [Google Scholar]
- Mc Conville, C.; Major, I.; Friend, D.R.; Clark, M.R.; Woolfson, A.D.; Malcolm, R.K. Development of polylactide and polyethylene vinyl acetate blends for the manufacture of vaginal rings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Barmpalexis, P.; Koutsidis, I.; Karavas, E.; Louka, D.; Papadimitriou, S.A.; Bikiaris, D.N. Development of PVP/PEG mixtures as appropriate carriers for the preparation of drug solid dispersions by melt mixing technique and optimization of dissolution using artificial neural networks. Eur. J. Pharm. Biopharm. 2013, 85, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; de Armas, H.N.; D’Autry, W.; Van Schepdael, A.; Van den Mooter, G. Characterization of ternary solid dispersions of Itraconazole in polyethylene glycol 6000/polyvidone-vinylacetate 64 blends. Eur. J. Pharm. Biopharm. 2008, 69, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.R.; Torrado Perez, A.R.; Roberson, D.A.; Shemelya, C.M.; MacDonald, E.; Wicker, R.B. Novel ABS-based binary and ternary polymer blends for material extrusion 3D printing. J. Mater. Res. 2014, 29, 1859–1866. [Google Scholar] [CrossRef]
- Roberson, D.; Shemelya, C.M.; MacDonald, E.; Wicker, R. Expanding the applicability of FDM-type technologies through materials development. Rapid Prototyp. J. 2015, 21, 137–143. [Google Scholar] [CrossRef]
- Siqueiros, J.G.; Schnittker, K.; Roberson, D.A. ABS-maleated SEBS blend as a 3D printable material. Virtual Phys. Prototyp. 2016, 11, 123–131. [Google Scholar] [CrossRef]
- Decker, N.; Yee, A. Assessing the use of binary blends of acrylonitrile butadiene styrene and post-consumer high density polyethylene in fused filament fabrication. Int. J. Addit. Subtrac. Mater. Manuf. 2017, 1, 161–171. [Google Scholar] [CrossRef]
- Cicala, G.; Ognibene, G.; Portuesi, S.; Blanco, I.; Rapisarda, M.; Pergolizzi, E.; Recca, G. Comparison of Ultem 9085 used in fused deposition modelling (FDM) with polytherimide blends. Materials 2018, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, Y.; Tang, Y.; Wang, B. Effects of styrene-acrylonitrile contents on the properties of ABS/SAN blends for fused deposition modeling. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Kosorn, W.; Sakulsumbat, M.; Uppanan, P.; Kaewkong, P.; Chantaweroad, S.; Jitsaard, J.; Sitthiseripratip, K.; Janvikul, W. PCL/PHBV blended three dimensional scaffolds fabricated by fused deposition modeling and responses of chondrocytes to the scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T.M. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Maeyaert, G.R.K. Bulk Compounding of PCL-PEO Blends for 3D Plotting of Scaffolds for Cardiovascular Tissue Engineering. J. Mater. Sci. Eng. 2013, 3, 3–6. [Google Scholar] [CrossRef]
- Lin, W.; Flanagan, D.R.; Linhardt, R.J. A novel fabrication of poly(ε-caprolactone) microspheres from blends of poly(ε-caprolactone) and poly(ethylene glycol) s. Polymer 1999, 40, 1731–1735. [Google Scholar] [CrossRef]
- Wu, D.C.; Loh, X.J.; Wu, Y.L.; Lay, C.L.; Liu, Y. “Living” controlled in situ gelling systems: Thiol-disulfide exchange method toward tailor-made biodegradable hydrogels. J. Am. Chem. Soc. 2010, 132, 15140–15143. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Widjaja, E.; Huang, Y.; Ng, X.W.; Loo, S.C.J.; Boey, F.Y.C.; Venkatraman, S.S. A New Insight for an Old System: Protein-PEG Colocalization in Relation to Protein Release from PCL/PEG Blends. Mol. Pharm. 2011, 8, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Capone, C.; Di Landro, L.; Inzoli, F.; Penco, M.; Sartore, L. Thermal and mechanical degradation during polymer extrusion processing. Polym. Eng. Sci. 2007, 47, 1813–1819. [Google Scholar] [CrossRef]
- Miller, E.; Rothstein, J.P. Control of the sharkskin instability in the extrusion of polymer melts using induced temperature gradients. Rheol. Acta 2004, 44, 160–173. [Google Scholar] [CrossRef]
- Venkataraman, N.; Rangarajan, S.; Matthewson, M.J.; Harper, B.; Safari, A.; Danforth, S.C.; Wu, G.; Langrana, N.; Guceri, S.; Yardimci, A. Feedstock material property-process relationships in fused deposition of ceramics (FDC). Rapid Prototyp. J. 2000, 6, 244–253. [Google Scholar] [CrossRef]
- Fischer, J.M. Handbook of Molded Part Shrinkage and Warpage; William Andrew: Amsterdam, The Netherlands, 2013; ISBN 1455730572. [Google Scholar]
- Turner, B.N.; Gold, S.A. A review of melt extrusion additive manufacturing processes: II. Materials, dimensional accuracy, and surface roughness. Rapid Prototyp. J. 2015, 21, 250–261. [Google Scholar] [CrossRef]
- Barlow, J.W.; Paul, D.R. Polymer blends and alloys—A review of selected considerations. Polym. Eng. Sci. 1981, 21, 985–996. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S. Dynamic mechanical properties of PLA/PHBV, PLA/PCL, PHBV/PCL blends and their nanocomposites with TiO2 as nanofiller. Thermochim. Acta 2015, 613, 41–53. [Google Scholar] [CrossRef]
- Verstraete, G.; Samaro, A.; Grymonpré, W.; Vanhoorne, V.; Van Snick, B.; Boone, M.N.; Hellemans, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef] [PubMed]
- ASTM International. ASTM D638-14 Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Espalin, D.; Alberto Ramirez, J.; Medina, F.; Johnson, W.M.; Rowell, M.; Deason, B.; Eubanks, M.; Turner, B.N.; Strong, R.; Gold, S.A. Rapid Prototyping Journal A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyp. J. 2014, 20, 192–204. [Google Scholar] [CrossRef]
- ASTM International. ASTM D2240-15e1 Standard Test Method for Rubber Property—Durometer Hardness; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Wang, S.; Capoen, L.; D’hooge, D.R.; Cardon, L. Can the melt flow index be used to predict the success of fused deposition modelling of commercial poly(lactic acid) filaments into 3D printed materials? Plast. Rubber Compos. 2018, 47, 9–16. [Google Scholar] [CrossRef]
- Shahriar, B.B.; France, C.; Valerie, N.; Arthur, C.; Christian, G. Toward improvement of the properties of parts manufactured by FFF (fused filament fabrication) through understanding the influence of temperature and rheological behaviour on the coalescence phenomenon. AIP Conf. Proc. 2017, 1896. [Google Scholar] [CrossRef]
- Ahn, S.; Montero, M.; Odell, D.; Roundy, S.; Wright, P.K. Anisotropic material properties of fused deposition modeling ABS. Rapid Prototyp. J. 2002, 8, 248–257. [Google Scholar] [CrossRef]
- Kaveh, M.; Badrossamay, M.; Foroozmehr, E.; Hemasian Etefagh, A. Optimization of the printing parameters affecting dimensional accuracy and internal cavity for HIPS material used in fused deposition modeling processes. J. Mater. Process. Technol. 2015, 226, 280–286. [Google Scholar] [CrossRef]
- Halidi, S.N.A.M.; Abdullah, J. Moisture effects on the ABS used for Fused Deposition Modeling rapid prototyping machine. In Proceedings of the Humanities, Science and Engineering Research (SHUSER), Kuala Lumpur, Malaysia, 24–27 June 2012. [Google Scholar] [CrossRef]
- Agarwala, M.K.; Jamalabad, V.R.; Langrana, N.A.; Safari, A.; Whalen, P.J.; Danforth, S.C. Structural quality of parts processed by fused deposition. Rapid Prototyp. J. 1996, 2, 4–19. [Google Scholar] [CrossRef]
- Wang, K. Die Swell of Complex Polymeric Systems. In Viscoelasticity—From Theory to Biological Applications; InTech: Houston, TX, USA, 2012. [Google Scholar]












| Name | Composition by Weight (%) | |||
|---|---|---|---|---|
| PVP-VA | P188 | PCL | PEO | |
| PVP-VA | 100 | - | - | - |
| PCL | - | - | 100 | - |
| F1 | 90 | 10 | - | - |
| F2 | 90 | - | 10 | - |
| F3 | 90 | - | - | 10 |
| F4 | 80 | - | 20 | - |
| F5 | 80 | - | - | 20 |
| F6 | 70 | - | 30 | - |
| F7 | 60 | - | 40 | - |
| F8 | 50 | - | 50 | - |
| F9 | 60 | - | 30 | 10 |
| F10 | 60 | 10 | 30 | - |
| F11 | 30 | - | 60 | 10 |
| Name | Composition by Weight (%) | |||
|---|---|---|---|---|
| PVP-VA | PCL | PEO | Caffeine | |
| DC 30% PVP-VA | 30 | 55 | 10 | 5 |
| DC 60% PVP-VA | 60 | 25 | 10 | 5 |
| 3DP 30% PVP-VA | 30 | 55 | 10 | 5 |
| 3DP 40% PVP-VA | 40 | −45 | 10 | 5 |
| 3DP 60% PVP-VA | 60 | 25 | 10 | 5 |
| Temperature (°C) | |||||||
|---|---|---|---|---|---|---|---|
| Zone 1 | Zone 2 | Zone 3 | Zone 4 | Zone 5 | Zone 6 | Flange | Die |
| 80 | 90 | 100 | 110 | 120 | 130 | 140 | 140 |
| Formulation | B (%Pa) (104) | εb (%) | E′ (Pa) |
|---|---|---|---|
| PVP-VA | 6.22 | 0.85 ± 0.19 | 1897.89 ± 2.27 |
| PCL | 0.35 | 59.07 ± 1.38 | 481.99 ± 0.04 |
| F1 | 8.33 | 0.68 ± 0.08 | 1768.03 ± 61.47 |
| F2 | 5.75 | 0.93 ± 0.12 | 1877.50 ± 19.19 |
| F3 | 2.10 | 2.34 ± 0.85 | 2033.35 ± 24.26 |
| F4 | 3.24 | 2.41 ± 0.67 | 1277.84 ± 2.76 |
| F5 | 1.89 | 2.29 ± 0.82 | 2314.50 ± 6.26 |
| F6 | 1.21 | 3.73 ± 2.28 | 2223.50 ± 59.54 |
| F7 | 0.10 | 78.58 ± 5.65 | 1295.80 ± 305.20 |
| F8 | 0.62 | 13.82 ± 5.34 | 1175.02 ± 34.18 |
| F9 | 0.15 | 54.46 ± 30.79 | 1223.47 ± 1.55 |
| F10 | 0.15 | 73.06 ± 4.15 | 935.16 ± 1.08 |
| F11 | 0.14 | 72.23 ± 6.67 | 995.94 ± 1.87 |
| Name | Extruder Torque | Melt Flow Rate at 140 °C | Melt Flow Rate at 150 °C |
|---|---|---|---|
| (%) | (g/10 min) | (g/10 min) | |
| PVP-VA | 40 | 0.00 ± 0.00 | 5.14 ± 0.12 |
| PCL | 10 | 11.10 ± 0.04 | 17.23 ± 0.77 |
| F1 | 15 | 4.51 ± 0.04 | 9.51 ± 0.17 |
| F2 | 20 | 3.01 ± 0.03 | 4.65 ± 0.70 |
| F3 | 15 | 2.33 ± 0.03 | 3.12 ± 0.30 |
| F4 | 20 | 2.89 ± 0.15 | 12.42 ± 0.41 |
| F5 | 15 | 0.55 ± 0.01 | 1.8 ± 0.01 |
| F6 | 15 | 4.74 ± 0.06 | 5.88 ± 0.15 |
| F7 | 10 | 6.88 ± 0.07 | 8.37 ± 0.04 |
| F8 | 10 | 7.06 ± 0.07 | 7.24 ± 0.05 |
| F9 | 10 | 3.56 ± 0.05 | 6.93 ± 0.07 |
| F10 | 10 | 9.30 ± 0.11 | 22.87 ± 0.69 |
| F11 | 10 | 7.52 ± 0.06 | 10.53 ± 0.02 |
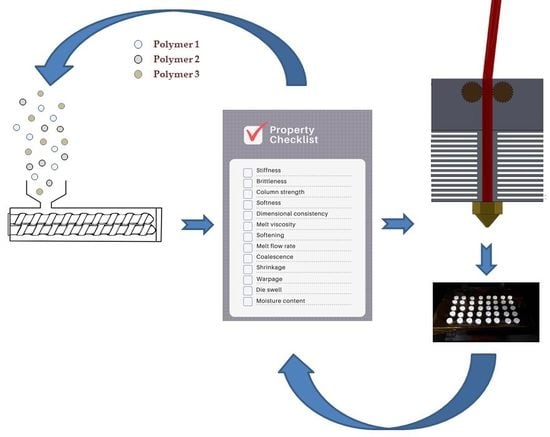
| Zone | Material Property | Comments |
|---|---|---|
| Feed | Filament stiffness | A very stiff filament will not permit winding onto spools. Therefore, the filament remains in the vertical axis and length will be limited by room height or other obstructions. Above a certain stiffness, feed length will be determined by height, which can self-support weight. For pre-screening, material stiffness can be measured in a number of different modes, tensile, flexural, or torsion. We utilized a DMA in the single cantilever, but a universal tester (tensile, flexural, and torsion) or a texture analyser can also be used [13,73]. Zhang et al. [13] allocated the breaking stress as a quantification of filament stiffness as tested by using a texture analyzer. |
| Filament brittleness | Brittle filaments can snap in the driving gears and prevent feeding. Brittleness (B) can be calculated from strain-at-break (εb) and storage modulus (E′) using the Brostow-Hagg Lobland-Narkis Equation (see Equation (2)) for brittleness [37]. Primarily elongation-at break (%) is the value calculated for εb and the values are obtained from tensile testing if the correct test specimens are available [74]. Our modified approach was to test filament lengths to obtain strain-at-break from 3-point bending directly. Others have performed similar tests but solely defined the strain-at-break data as a brittleness measurement [13,73]. We calculate that B should be less than 0.0002 %Pa for materials to make suitable filaments. | |
| Column strength | Since most filaments act as a piston on the melt-front in the liquefier, the ability of the filament to withstand compressive force without buckling is an important variable [68,75]. Venkataraman et al. [68] determined a critical ratio for ceramic-based filaments above which a filament will withstand buckling. The ratio states that, if the elastic modulus of the filament is greater than the apparent viscosity by 3–5 × 105 s−1 then the filament will maintain sufficient column strength during printing. Most thermoplastic materials will maintain the critical ratio [75], but it is a useful pre-screening tool for untypical materials or highly-filled materials. | |
| Filament softness | Soft materials can be squeezed between driving gears, which would limite or prevent feeding. Material hardness can be measured a number of ways, but the Shore durometer method is the most common approach [76]. | |
| Dimensional consistency | Filament consistency will determine the feed rate to the heating end. Consistency is more than just a measure of filament diameter and can include ovality, pockmarks, gaps, and general deformities. Visual inspection is sufficient for eliminating the majority of the irregular filament. | |
| Filament diameter | Diameter ultimately determines feed rate to the heating end. Inconsistent filament diameter will result in inconsistent deposition and poor prints. Extrusion flow surging is a problem that occurs due to fluctuations in the feed or transition zone in the extrusion process [45]. A melt pump will eradicate the problem and produce a uniform filament but at added capital cost. Consistent material feeding and a correct temperature profile that permits stable melt formation can eliminate most surging. Die design can reduce the phenomenon and a longer land length by promoting a consistent melt output. Filament diameter is best measured at the point of filament production using laser micrometers or ultrasonic gauges. | |
| Hot | Melt viscosity | As material softens and begins to melt, feeding from the melt to the nozzle is dependent on the back pressure formed due to the action of the driving gears forcing the filament downwards. High viscosity and the back pressure will be insufficient to force the melt through the nozzle die. Too high a force can lead to buckling of the filament [68]. Low viscosity and too much material will be pushed through the nozzle by preventing proper deposition. Melt viscosity is determined by a rheometer. A capillary rheometer at low shear is best suited since it most closely resembles the FFF extruder setup. |
| Softening | Filament entering past the driving gear acts as a piston on the molten polymer below and, therefore, must maintain sufficient stiffness before melting to create the required back pressure. If the filament softens too soon, piston action efficiency will decrease and hinder melt deposition. A DMA storage modulus curve is a good representation of the stiffness of the material over an elevated temperature range. | |
| Deposition | Melt flow rate | Melt flow rate is related to viscosity and is temperature dependent. High flow rate materials will more easily be pushed through the liquefier and nozzle. Too high and melt deposition will be uncontrollable. Low flow rate materials will be harder to push through the liquefier and nozzle. Too low of a flow rate and melt deposition becomes unachievable. The melt flow rate is determined by a melt flow indexer. Wang et al. [77] have recently determined that commercial filament grades should be greater than 10 g/10 min to achieve acceptable print quality. |
| Melt feed consistency | The homogeneous flow of material is a critical necessity for a successful 3DP part. Surge feeding or starvation of material result in imperfection in the part’s building process. Most common signs of feed inconsistency are missing layers, layers misalignment, weak infill, low dimensional accuracy, and layer splitting. Feedstock material with consistent dimensions is crucial. | |
| Coalescence | Poor layer coalescence leads to inconsistencies in the structure of the printed parts, which creates critical points of failure, poor performance, and geometrical discrepancies. Coalescence increases with decreases in melt viscosity since there is greater polymer chain mobility and intermingling between layers [78]. Therefore, poor interlayer adhesion may be improved through higher printing temperatures. If deposited layers fail to adhere, print quality suffers considerably. Finished parts with the strong layer-to-layer union will possess higher mechanical toughness [79]. | |
| Shrinkage and Warpage | Parts with subpar adhesion to the printing bed could exhibit warping due to deposited layers cooling down and contracting because of internal stresses, which results in partial deformation. If material fails to stick properly to the printing bed, a higher printing bed temperature might be necessary. The use of Kapton tape or Scotch™ blue painters tape improves the adhesion of materials to the printing bed and protects the bed from scratches. Environmental conditions, such as room temperature, should be taken into consideration when dealing with poor adhesion or warping since thermal gradients are the primary cause of internal stress [70]. Correction factors can be applied at the design stage to accommodate for known print shrinkage of specific materials. These factors are prevalent for common materials and are a common feature of 3D printing software. Kaveh 2015 et al. [80] describe a means for determining correction factors for material through the printing of a series of cubes, cylinders, and stairs. | |
| Moisture content | Trapped water will evaporate by exiting the nozzle and creating bubbles inside the extruded material, which disrupts the steady deposition of layers [81]. When using hygroscopic materials for long printing processes, it is important to consider the storage conditions of the feedstock material used for manufacturing. Production could fail due to absorption of moisture by the material. Adequate drying procedures should be adopted for an improperly stored filament. | |
| Die swell | Die swell is a well-established issue in polymer extrusion. The phenomena relates to the exiting diameter of the extrudate being greater than the diameter of the die and is related to the viscoelastic nature of the polymer. Die swell increases with increasing polymer molecular weight. It will affect the quality of the final print since it reduces the dimensional accuracy of the deposited layer. Die swell from the liquefier nozzle may be reduced through changes to the material formulation or changes in the nozzle design. However, the short land length of FFF printer nozzles may preclude the latter option. The primary means of dealing with die swell is to accommodate the design by specifying the deposited layer thickness to be 1.2–1.5 times the nozzle die diameter [82]. Material die swell can be measured using a capillary die rheometer [83]. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuenmayor, E.; Forde, M.; Healy, A.V.; Devine, D.M.; Lyons, J.G.; McConville, C.; Major, I. Material Considerations for Fused-Filament Fabrication of Solid Dosage Forms. Pharmaceutics 2018, 10, 44. https://doi.org/10.3390/pharmaceutics10020044
Fuenmayor E, Forde M, Healy AV, Devine DM, Lyons JG, McConville C, Major I. Material Considerations for Fused-Filament Fabrication of Solid Dosage Forms. Pharmaceutics. 2018; 10(2):44. https://doi.org/10.3390/pharmaceutics10020044
Chicago/Turabian StyleFuenmayor, Evert, Martin Forde, Andrew V. Healy, Declan M. Devine, John G. Lyons, Christopher McConville, and Ian Major. 2018. "Material Considerations for Fused-Filament Fabrication of Solid Dosage Forms" Pharmaceutics 10, no. 2: 44. https://doi.org/10.3390/pharmaceutics10020044
APA StyleFuenmayor, E., Forde, M., Healy, A. V., Devine, D. M., Lyons, J. G., McConville, C., & Major, I. (2018). Material Considerations for Fused-Filament Fabrication of Solid Dosage Forms. Pharmaceutics, 10(2), 44. https://doi.org/10.3390/pharmaceutics10020044