Electrically and Ultrasonically Enhanced Transdermal Delivery of Methotrexate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
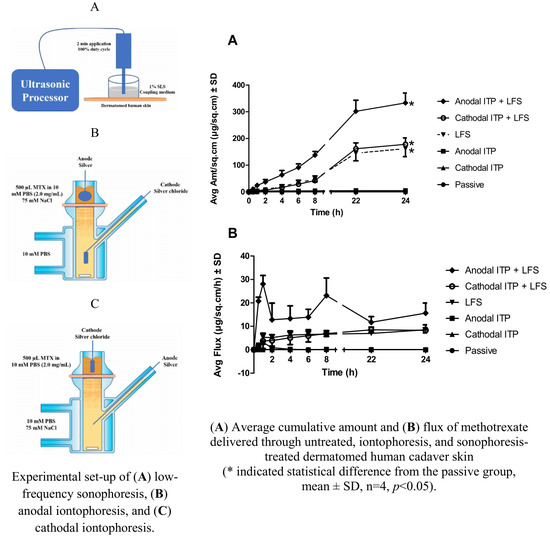
2.2. Experimental Conditions and Apparatus
2.3. Dye Binding Studies
2.4. Skin Integrity Measurement
2.5. In Vitro Permeation Studies using Vertical Franz Diffusion Cells
2.6. Skin Distribution Studies
2.7. Quantitative Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Dye Binding Studies
3.2. Skin Integrity Measurement
3.3. In Vitro Permeation Studies Using Vertical Franz Diffusion Cells
3.3.1. Passive Permeation
3.3.2. ITP-Mediated Delivery of MTX
3.3.3. Sonophoresis-Mediated Delivery of MTX
3.3.4. The Combination of ITP and Sonophoresis
3.3.5. Steady-State Plasma Concentration of MTX
3.4. Skin Distribution Studies
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| DI water | Deionized water |
| ITP | Iontophoresis |
| LFS | Low-frequency sonophoresis |
| MTX | Methotrexate |
| OCT | Optimal cutting temperature |
| PBS | Phosphate Buffer Saline |
| RP-HPLC | Reversed-Phase High-Performance Liquid Chromatography |
| SD | Standard Deviation |
| SLS | Sodium Lauryl Sulfate |
| TEWL | Transepidermal Water Loss |
References
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ita, K.B. Transdermal drug delivery: Progress and challenges. J. Drug Deliv. Sci. Technol. 2014, 24, 245–250. [Google Scholar] [CrossRef]
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Boucaud, A.; Montharu, J.; Machet, L.; Arbeille, B.; Machet, M.C.; Patat, F.; Vaillant, L. Clinical, histologic, and electron microscopy study of skin exposed to low-frequency ultrasound. Anat. Rec. 2001, 264, 114–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Banga, A.K. Microneedle-mediated delivery of vismodegib across skin. J. Investig. Dermatol. 2015, 135, S58. [Google Scholar]
- Nguyen, H.X.; Puri, A.; Banga, A.K. Methods to simulate rubbing of topical formulation for in vitro skin permeation studies. Int. J. Pharm. 2017, 519, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In vitro–in vivo correlation in skin permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Herwadkar, A.; Sachdeva, V.; Taylor, L.F.; Silver, H.; Banga, A.K. Low frequency sonophoresis mediated transdermal and intradermal delivery of ketoprofen. Int. J. Pharm. 2012, 423, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Banga, A.K. Enhanced skin delivery of vismodegib by microneedle treatment. Drug Deliv. Transl. Res. 2015, 5, 407–423. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Banga, A.K. Fabrication, characterization and application of sugar microneedles for transdermal drug delivery. Ther. Deliv. 2017, 8, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Tas, C.; Joyce, J.C.; Nguyen, H.X.; Eangoor, P.; Knaack, J.S.; Banga, A.K.; Prausnitz, M.R. Dihydroergotamine mesylate-loaded dissolving microneedle patch made of polyvinylpyrrolidone for management of acute migraine therapy. J. Controll. Release 2017, 268, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, M.; Ueda, H.; Nakamura, Y.; Hirayama, K.; Atobe, M.; Kobayashi, D.; Morimoto, Y. Characterization of transdermal solute transport induced by low-frequency ultrasound in the hairless rat skin. J. Controll. Release 2003, 92, 137–146. [Google Scholar] [CrossRef]
- Polat, B.E.; Hart, D.; Langer, R.; Blankschtein, D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J. Controll. Release 2011, 152, 330–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bommannan, D.; Menon, G.K.; Okuyama, H.; Elias, P.M.; Guy, R.H. Sonophoresis. II. Examination of the mechanism (s) of ultrasound-enhanced transdermal drug delivery. Pharm. Res. 1992, 9, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Kost, J. Low-Frequency Sonophoresis: A Noninvasive Method of Drug Delivery and Diagnostics. Biotechnol. Prog. 2000, 16, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Park, H.; Seo, J.; Lee, S. Sonophoresis in transdermal drug deliverys. Ultrasonics 2014, 54, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, C.C.J.; Blankschtein, D.; Langer, R. An investigation of the role of cavitation in low-frequency ultrasound-mediated transdermal drug transport. Pharm. Res. 2002, 19, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Mutoh, M.; Seki, T.; Kobayashi, D.; Morimoto, Y. Acoustic cavitation as an enhancing mechanism of low-frequency sonophoresis for transdermal drug delivery. Biol. Pharm. Bull. 2009, 32, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Katikaneni, S.; Li, G.; Badkar, A.; Banga, A.K. Transdermal delivery of a approximately 13 kDa protein—An in vivo comparison of physical enhancement methods. J. Drug Target. 2010, 18, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Blankschtein, D.; Langer, R. An explanation for the variation of the sonophoretic transdermal transport enhancement from drug to drug. J. Pharm. Sci. 1997, 86, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Recent progress in transdermal sonophoresis. Pharm. Dev. Technol. 2017, 22, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Banga, A.K. Electrically Assisted Transdermal and Topical Drug Delivery; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Alvarez-Figueroa, M.J.; Delgado-Charro, M.B.; Blanco-Mendez, J. Passive and iontophoretic transdermal penetration of methotrexate. Int. J. Pharm. 2001, 212, 101–107. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Kumar, B.R.; Udupa, N.; Balachandran, C. Topical methotrexate delivered by iontophoresis in the treatment of recalcitrant psoriais-a case report. Int. J. Dermatol. 2003, 42, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Vemulapalli, V.; Yang, Y.; Friden, P.M.; Banga, A.K. Synergistic effect of iontophoresis and soluble microneedles for transdermal delivery of methotrexate. J. Pharm. Pharmacol. 2008, 60, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Chladek, J.; Martinkova, J.; Šimková, M.; Vaněčková, J.; Koudelkova, V.; Nožičková, M. Pharmacokinetics of low doses of methotrexate in patients with psoriasis over the early period of treatment. Eur. J. Clin. Pharmacol. 1998, 53, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Vemulapalli, V.; Banga, A.K.; Friden, P.M. Optimization of iontophoretic parameters for the transdermal delivery of methotrexate. Drug Deliv. 2008, 15, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Figueroa, M.J.; Blanco-Mendez, J. Transdermal delivery of methotrexate: Iontophoretic delivery from hydrogels and passive delivery from microemulsions. Int. J. Pharm. 2001, 215, 57–65. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Banga, A.K. Delivery of methotrexate and characterization of skin treated by fabricated PLGA microneedles and fractional ablative laser. Pharm. Res. 2018, 35, 68. [Google Scholar] [CrossRef] [PubMed]
- Le, L.; Kost, J.; Mitragotri, S. Combined effect of low-frequency ultrasound and iontophoresis: Applications for transdermal heparin delivery. Pharm. Res. 2000, 17, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Bozorg, B.D.; Kim, Y.; Wieber, A.; Birk, G.; Lubda, D.; Banga, A.K. Poly (vinyl alcohol) microneedles: Fabrication, characterization, and application for transdermal drug delivery of doxorubicin. Eur. J. Pharm. Biopharm. 2018, 129, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Nguyen, H.X.; Banga, A.K. Microneedle-mediated intradermal delivery of epigallocatechin-3-gallate. Int. J. Cosmet. Sci. 2016, 38, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Puri, A.; Bhattaccharjee, S.A.; Banga, A.K. Qualitative and quantitative analysis of lateral diffusion of drugs in human skin. Int. J. Pharm. 2018, 544, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, A.; Ganti, S.S.; Nguyen, H.X.; Birk, G.; Wieber, A.; Lubda, D.; Banga, A.K. Development and evaluation of a polyvinyl alcohol based topical gel. J. Drug Deliv. Sci. Technol. 2017, 39, 210–216. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Banga, A.K. Effect of ablative laser on in vitro transungual delivery. Int. J. Pharm. 2018, 544, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Hinsberg, C.-V.; Ine, W.H.M.; Verhoef, J.; Spies, F.; Bouwstra, J.A.; Gooris, G.S.; Junginger, H.E.; Boddé, H.E. Electroperturbation of the human skin barrier in vitro: II. Effects on stratum corneum lipid ordering and ultrastructure. Microsc. Res. Tech. 1997, 37, 200–213. [Google Scholar] [CrossRef]
- Prasad, R.; Koul, V.; Anand, S.; Khar, R.K. Effect of DC/mDC iontophoresis and terpenes on transdermal permeation of methotrexate: In vitro study. Int. J. Pharm. 2007, 333, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kost, J.; Leong, K.; Langer, R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc. Natl. Acad. Sci. USA 1989, 86, 7663–7666. [Google Scholar] [CrossRef] [PubMed]
- Pires-de-Campos, M.S.M.; Leonardi, G.R.; Chorilli, M.; Spadari-Bratfisch, R.C.; Polacow, M.L.O.; Grassi-Kassisse, D.M. The effect of topical caffeine on the morphology of swine hypodermis as measured by ultrasound. J. Cosmet. Dermatol. 2008, 7, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Tachibana, K.; Ogawa, K.; Tsujita, N.; Tomita, A. Scanning electron microscopic evaluation of the skin surface after ultrasound exposure. Anat. Rec. 1997, 247, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Hikima, T.; Hirai, Y.; Tojo, K. Effect of ultrasound application on skin metabolism of prednisolone 21-acetate. Pharm. Res. 1998, 15, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Farrell, J.; Tang, H.; Terahara, T.; Kost, J.; Langer, R. Determination of threshold energy dose for ultrasound-induced transdermal drug transport. J. Controll. Release 2000, 63, 41–52. [Google Scholar] [CrossRef]
- Kalia, Y.N.; Nonato, L.B.; Guy, R.H. The effect of iontophoresis on skin barrier integrity: Non-invasive evaluation by impedance spectroscopy and transepidermal water loss. Pharm. Res. 1996, 13, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Tezel, A.; Dokka, S.; Kelly, S.; Hardee, G.E.; Mitragotri, S. Topical delivery of anti-sense oligonucleotides using low-frequency sonophoresis. Pharm. Res. 2004, 21, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Kalia, Y.N.; Alberti, I.; Sekkat, N.; Curdy, C.; Naik, A.; Guy, R.H. Normalization of stratum corneum barrier function and transepidermal water loss in vivo. Pharm. Res. 2000, 17, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Yoshihara, T.; Yamamoto, M.; Konno, K.; Momoi, Y.; Nishifuji, K.; Iwasaki, T. Transepidermal water loss (TEWL) reflects skin barrier function of dog. J. Vet. Med. Sci. 2008, 70, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Blankschtein, D.; Langer, R. Transdermal drug delivery using low-frequency sonophoresis. Pharm. Res. 1996, 13, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, G.D.; McCullough, J.L.; Olsen, E. Topical methotrexate therapy for psoriasis. Arch. Dermatol. 1989, 125, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Pikal, M.J. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 2001, 46, 281–305. [Google Scholar] [CrossRef]
- Singh, J.; Singh, S. Transdermal iontophoresis: Effect of penetration enhancer and iontophoresis on drug transport and surface characteristics of human epidermis. In Exogenous Dermatology; Karger Publishers: Basel, Switzerland, 1995; Volume 22, pp. 179–183. [Google Scholar]
- Prasad, R.; Anand, S.; Khar, R.K.; Dinda, A.K.; Koul, V. Studies on in vitro and in vivo transdermal flux enhancement of methotrexate by a combinational approach in comparison to oral delivery. Drug Dev. Ind. Pharm. 2009, 35, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Stagni, G.; Shukla, C. Pharmacokinetics of methotrexate in rabbit skin and plasma after iv-bolus and iontophoretic administrations. J. Controll. Release 2003, 93, 283–292. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Srinivasan, S.; Barman, R.; Mo, S.H.; Polat, B.E.; Langer, R.; Blankschtein, D. Applicability and safety of dual-frequency ultrasonic treatment for the transdermal delivery of drugs. J. Controll. Release 2015, 202, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banga, A.K. New technologies to allow transdermal delivery of therapeutic proteins and small water-soluble drugs. Am. J. Drug Deliv. 2006, 4, 221–230. [Google Scholar] [CrossRef]
- Wolloch, L.; Kost, J. The importance of microjet vs shock wave formation in sonophoresis. J. Controll. Release 2010, 148, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Blankschtein, D.; Langer, R. Ultrasound-mediated transdermal protein delivery. Science 1995, 269, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Boucaud, A.; Garrigue, M.A.; Machet, L.; Vaillant, L.; Patat, F. Effect of sonication parameters on transdermal delivery of insulin to hairless rats. J. Controll. Release 2002, 81, 113–119. [Google Scholar] [CrossRef]
- Tezel, A.; Mitragotri, S. Interactions of inertial cavitation bubbles with stratum corneum lipid bilayers during low-frequency sonophoresis. Biophys. J. 2003, 85, 3502–3512. [Google Scholar] [CrossRef]
- Zorec, B.; Jelenc, J.; Miklavčič, D.; Pavšelj, N. Ultrasound and electric pulses for transdermal drug delivery enhancement: Ex vivo assessment of methods with in vivo oriented experimental protocols. Int. J. Pharm. 2015, 490, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Schoellhammer, C.M.; Polat, B.E.; Mendenhall, J.; Maa, R.; Jones, B.; Hart, D.P.; Langer, R.; Blankschtein, D. Rapid skin permeabilization by the simultaneous application of dual-frequency, high-intensity ultrasound. J. Controll. Release 2012, 163, 154–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucaud, A.; Machet, L.; Arbeille, B.; Machet, M.C.; Sournac, M.; Mavon, A.; Patat, F.; Vaillant, L. In vitro study of low-frequency ultrasound-enhanced transdermal transport of fentanyl and caffeine across human and hairless rat skin. Int. J. Pharm. 2001, 228, 69–77. [Google Scholar] [CrossRef]
- Mitragotri, S.; Kost, J. Transdermal delivery of heparin and low-molecular weight heparin using low-frequency ultrasound. Pharm. Res. 2001, 18, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.B.; Lee, S.; Maione, E.; Roy, R.B.; McElligott, S.; Shung, K.K. Ultrasound-mediated transdermal transport of insulin in vitro through human skin using novel transducer designs. Ultrasound Med. Biol. 2003, 29, 311–317. [Google Scholar] [CrossRef]
- Tezel, A.; Sens, A.; Mitragotri, S. Investigations of the role of cavitation in low-frequency sonophoresis using acoustic spectroscopy. J. Pharm. Sci. 2002, 91, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Shirouzu, K.; Nishiyama, T.; Hikima, T.; Tojo, K. Synergistic effect of sonophoresis and iontophoresis in transdermal drug delivery. J. Chem. Eng. Jpn. 2008, 41, 300–305. [Google Scholar] [CrossRef]
- Hikima, T.; Ohsumi, S.; Shirouzu, K.; Tojo, K. Mechanisms of synergistic skin penetration by sonophoresis and iontophoresis. Biol. Pharm. Bull. 2009, 32, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wei, J.; Iliescu, C. Sonophoretic enhanced microneedles array (SEMA)—Improving the efficiency of transdermal drug delivery. Sens. Actuators B Chem. 2010, 145, 54–60. [Google Scholar] [CrossRef]
- Guy, R.H.; Hadgraft, J. Rate control in transdermal drug delivery? Int. J. Pharm. 1992, 82, R1–R6. [Google Scholar] [CrossRef]
- Iqbal, M.P.; Baig, J.A.; Ali, A.A.; Niazi, S.K.; Mehboobali, N.; Hussain, M.A. The effects of non-steroidal anti-inflammatory drugs on the disposition of methotrexate in patients with rheumatoid arthritis. Biopharm. Drug Dispos. 1998, 19, 163–167. [Google Scholar] [CrossRef]
- Morimoto, Y.; Mutoh, M.; Ueda, H.; Fang, L.; Hirayama, K.; Atobe, M.; Kobayashi, D. Elucidation of the transport pathway in hairless rat skin enhanced by low-frequency sonophoresis based on the solute–water transport relationship and confocal microscopy. J. Controll. Release 2005, 103, 587–597. [Google Scholar] [CrossRef] [PubMed]





| Compartment | Passive a | Anodal ITP b | Cathodal ITP c | LFS d | Anodal ITP b + LFS d | Cathodal ITP c + LFS d |
|---|---|---|---|---|---|---|
| Donor | 500 µL MTX 2.0 mg/mL in 10 mM PBS | 500 µL MTX 2.0 mg/mL in 10 mM PBS (75 mM NaCl f) | 500 µL MTX 2.0 mg/mL in 10 mM PBS | 500 µL MTX 2.0 mg/mL in 10 mM PBS | 500 µL MTX 2.0 mg/mL in 10 mM PBS (75 mM NaCl f) | 500 µL MTX 2.0 mg/mL in 10 mM PBS |
| Receptor | 5 mL PBS (10 mM) | 5 mL PBS (10 mM) | 5 mL PBS (10 mM, 75 mM NaCl f) | 5 mL PBS (10 mM) | 5 mL PBS (10 mM) | 5 mL PBS (10 mM, 75 mM NaCl f) |
| Skin pretreatment | NA e | NA e | NA e | Two-min LFS, 100% duty cycle | Two-min LFS, 100% duty cycle | Two-min LFS, 100% duty cycle |
| Group | Q24a (µg/sq·cm) | Lag Time b (h) | Jss (µg/sq·cm/h) c | Kp (cm/h) d × 10−4 | Css (ng/mL) e |
|---|---|---|---|---|---|
| Passive | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Anodal ITP | 4.74 ± 0.62 | 3.57 ± 1.07 | 0.05 ± 0.03 | 0.26 ± 0.14 | 0.28 ± 0.15 |
| Cathodal ITP | 0.54 ± 0.07 | 0.20 ± 0.05 | 0.01 ± 0.00 | 0.07 ± 0.02 | 0.08 ± 0.02 |
| LFS | 161.92 ± 30.06 | −5.09 ± 1.03 | 6.81 ± 1.31 | 34.04 ± 6.55 | 36.92 ± 7.11 |
| Anodal ITP + LFS | 333.10 ± 37.01 | 11.83 ± 10.77 | 13.42 ± 1.95 | 67.08 ± 9.73 | 72.77 ± 10.55 |
| Cathodal ITP + LFS | 178.30 ± 23.79 | −15.93 ± 16.74 | 8.03 ± 1.17 | 40.14 ± 5.85 | 43.54 ± 6.35 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.X.; Banga, A.K. Electrically and Ultrasonically Enhanced Transdermal Delivery of Methotrexate. Pharmaceutics 2018, 10, 117. https://doi.org/10.3390/pharmaceutics10030117
Nguyen HX, Banga AK. Electrically and Ultrasonically Enhanced Transdermal Delivery of Methotrexate. Pharmaceutics. 2018; 10(3):117. https://doi.org/10.3390/pharmaceutics10030117
Chicago/Turabian StyleNguyen, Hiep X., and Ajay K. Banga. 2018. "Electrically and Ultrasonically Enhanced Transdermal Delivery of Methotrexate" Pharmaceutics 10, no. 3: 117. https://doi.org/10.3390/pharmaceutics10030117
APA StyleNguyen, H. X., & Banga, A. K. (2018). Electrically and Ultrasonically Enhanced Transdermal Delivery of Methotrexate. Pharmaceutics, 10(3), 117. https://doi.org/10.3390/pharmaceutics10030117