Combined Use of N-Palmitoyl-Glycine-Histidine Gel and Several Penetration Enhancers on the Skin Permeation and Concentration of Metronidazole
Abstract
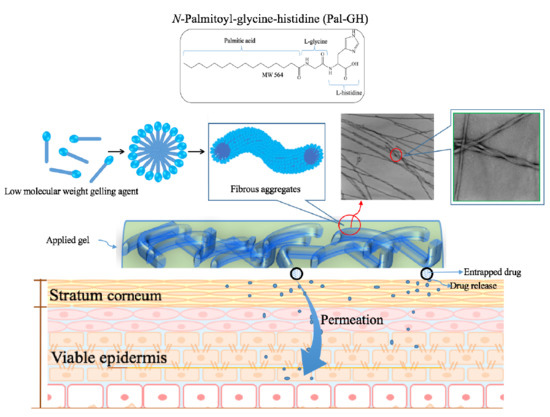
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of Applied Formulations
2.4. In Vitro Skin Permeation Experiment
2.5. In Vitro Release Experiment
2.6. Evaluation of Rheological Properties for N-Palmitoyl-Glycine-Histidine (Pal-GH) Formulations
2.7. Skin Concentration of Metronidazole (MTZ)
2.8. Determination of MTZ
2.9. Small Angle X-ray Scattering Analysis (SAXS)
2.10. Transmission Electron Microscopy (TEM)
2.11. Statistical Analysis
3. Results
3.1. Physical Properties of Pal-GH Gel Formulation
3.2. In Vitro Skin Permeation of MTZ from Aqueous Solution
3.3. In Vitro Release and Skin Permeation of MTZ from Pal-GH Gels
3.4. Skin Disposition of MTZ after Topical Application of Pal-GH Formulations
3.5. Thixotropic Properties of Pal-GH Gel Formulations
3.6. TEM Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Sabrina, D.; Futaki, M.; Okada, A.; Kadhum, W.R.; Todo, H.; Sugibayashi, K. Design of a topically applied gel spray formulation with ivermectin using a novel low molecular weight gelling agent, palmitoyl-glycine-histidine, to treat scabies. Chem. Pharm. Bull. 2018, 66, 1–8. [Google Scholar] [CrossRef]
- Watanabe, T.; Hasegawa, T.; Takahashi, H.; Ishibashi, T.; Takayama, K.; Sugibayashi, K. Utility of three-dimensional cultured human skin model as a tool to evaluate skin permeation of drugs. Altern. Anim. Test Exp. 2011, 8, 1–14. [Google Scholar]
- Morimoto, Y.; Hatanaka, T.; Sugibayashi, K.; Omiya, H. Prediction of skin permeability of drugs: comparison of human and hairless rat skin. J. Pharm. Pharmacol. 1992, 44, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for testing of transdermal drugs. Clin. Pharmacol. Adv. Appl. 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.C.; Maibach, H.I. Animal models for percutaneous absorption. J. Appl. Toxicol. 2015, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Roberts, M.S. The effect of occlusion on the epidermal penetration of parabens from a commercial test ointment, acetone and ethanol vehicles. J. Investig. Dermatol. 2000, 115, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Asbill, C.S.; Michniak, B.B. Percutaneous penetration enhancers: Local versus transdermal activity. Pharm. Sci. Technol. 2000, 3, 36–41. [Google Scholar] [CrossRef]
- Asbill, C.S.; El-Kattan, A.F.; Michniak, B. Enhancement of transdermal drug delivery: Chemical and physical approaches. Crit. Rev. Ther. Drug Carrier Syst. 2000, 17, 621–658. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C.W.; Hadgraft, J.W.; Caron, G.A.; Sarkany, I. The effect of particle size on the percutaneous absorption of fluocinolon acetonide. Br. J. Dermatol. 1965, 77, 575–578. [Google Scholar] [CrossRef]
- Coldman, M.F.; Poulsen, B.J.; Higuchi, T. Enhancement of percutaneous absorption by the use of volatile: nonvolatile system as vehicles. J. Pharm. Sci. 1969, 58, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Hoelgaard, A.; Mollgaard, B. Dermal drug delivery-improvement by choice of vehicle or drug derivative. J. Control. Release 1985, 2, 111–120. [Google Scholar] [CrossRef]
- Nicolazzo, J.A.; Morgan, T.M.; Reed, B.L.; Finnin, B.C. Synergistic enhancement of testosterone transdermal delivery. J. Control. Release 2005, 103, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Kurihara-Bergstrom, T.; Knutson, K.; DeNoble, L.; Goates, C.Y. Percutaneous absorption enhancement of an ionic molecule by ethanol-water systems in human skin. Pharm. Res. 1990, 7, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Leopold, C.S.; Lippold, B.C. An attempt to clarify the mechanism of the penetration enhancing effects of lipophilic vehicles with differential scanning calorimetry (DSC). J. Pharm. Pharmacol. 1995, 47, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.E.; Watkinson, A.C.; Green, D.M.; Hadgraft, J.; Brain, K. The relative effect of azone and transcutol on permeant diffusivity and solubility in human stratum corneum. Pharm. Res. 1996, 13, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.H.; Fuller, P. Diclofenac sodium topical solution 1.5% w/w with dimethyl sulphoxide compared with placebo for the treatment of osteoarthritis; pooledsafety results. Postgrad. Med. 2011, 123, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Thong, H.Y.; Zhai, H.; Maibach, H.I. Percutaneous penetration enhancers: An overview. Skin Pharmacol. Physiol. 2007, 20, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Ali, A.; Aqil, M.; Ahad, A. Transdermal drug delivery of labetalol hydrochloride: Feasibility and effect of penetration enhancers. J. Pharm. Bioallied. Sci. 2010, 2, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Marren, K. Dimethyl sulfoxide: An effective penetration enhancer for topical administration of NSAIDs. Phys. Sportsmed. 2011, 39, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Scheuplein, R.J.; Blank, I.H. Permeability of the skin. Physiol. Rev. 1971, 51, 702–747. [Google Scholar] [CrossRef] [PubMed]
- Sekura, D.L.; Scala, J. The percutaneous absorption of alkyl methyl sulfoxides. Adv. Biol. Skin. 1972, 12, 257–269. [Google Scholar] [PubMed]
- Mills, P.C. Vehicle effects on the in vitro penetration of testosterone through equine skin. Vet. Res. Commun. 2007, 31, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Gee, C.M.; Watkinson, A.C.; Nicolazzo, J.A.; Finnin, B.C. The effect of formulation excipients on the penetration and lateral diffusion of ibuprofen on and within the stratum corneum following topical application to humans. J. Pharm. Sci. 2014, 103, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Kietzmann, M.; Blume, B. Percutaneus absorption of betamethasone from different formulations using the isolated perfused bovine udder. In Vitro Toxicol. 1997, 10, 11–15. [Google Scholar]
- Herai, H.; Gratieri, T.; Thomazine, J.A.; Bentley, M.V.; Lopez, R.F. Doxorubicin skin penetration from monoolein-containing propylene glycol formulations. Int. J. Pharm. 2007, 32, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Mollgaard, B.; Hoelgaard, A. Vehicle effect on topical drug delivery. I. Influence of glycols and drug concentration on skin transport. Acta Pharm. Suec. 1983, 20, 433–442. [Google Scholar] [PubMed]
- Hirvonen, J.; Rajala, R.; Vihervaara, P.; Laine, E.; Paronen, P.; Urtti, A. Mechanism and reversibility of penetration enhancer action in the skin. Eur. J. Pharm. Biopharm. 1994, 40, 81–85. [Google Scholar]
- Anderson, R.L.; Cassidy, J.M. Variation in physical dimension and chemical composition of human stratum corneum. J. Investig. Dermatol. 1973, 61, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Shundo, A.; Ohno, M.; Fujita, S.; Saruhashi, K.; Miyachi, N.; Miyaji, K.; Tanaka, K. Modulation of physical properties of supramolecular hydrogels based on a hydrophobic core. Phys. Chem. Chem. Phys. 2015, 17, 2192–2198. [Google Scholar] [CrossRef] [PubMed]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta 2009, 11, 2362–2373. [Google Scholar] [CrossRef] [PubMed]
- Mallia, V.A.; Weiss, R.G. Correlations between thixotropic and structural properties of molecular gels with crystalline networks. Soft Matter. 2016, 12, 3665–3676. [Google Scholar] [CrossRef] [PubMed]
- Sugibayashi, K.; Todo, H.; Oshizaka, T.; Owada, Y. Mathematical model to predict skin concentration of drugs: Toward utilization of silicone membrane to predict skin concentration of drugs as an animal testing alternative. Pharm. Res. 2010, 27, 134–142. [Google Scholar] [CrossRef] [PubMed]








| Formulation Code | Pal-GH Conc. (Premix) | Chemical Penetration Enhancer |
|---|---|---|
| F2.5MTZ | 2.5% | No enhancer |
| F5MTZ | 5% | No enhancer |
| F5IPM-MTZ | IPM 10% | |
| F5PG-MTZ | Propylene glycol 4% | |
| F5DMSO-MTZ | DMSO 5% | |
| F5EtOH-MTZ | Ethanol 20% | |
| F5TRANS-MTZ | isopropyl myristate 10% | |
| F10MTZ | 10% | No enhancer |
| Aqueous solution | No enhancer | |
| IPMMTZ | IPM 10% | |
| PGMTZ | Propylene glycol 4% | |
| DMSOMTZ | DMSO 5% | |
| EtOHMTZ | Ethanol 20% | |
| TRANSMTZ | Transcutol 10% |
| Formulation Code | Release Rate (K) (µmol/cm2/h0.5) |
|---|---|
| F5MTZ | 4.99 ± 0.99 |
| F5IPM-MTZ | 5.07 ± 0.09 |
| F5PG-MTZ | 4.43 ± 0.07 |
| F5DMSO-MTZ | 3.53 ± 0.05 |
| F5EtOH-MTZ | 1.11 ± 0.09 |
| F5TRANS-MTZ | 3.06 ± 0.11 |
| Formulation Code | Flux (µg/cm2/h) | Permeability Coefficient (×10−7 cm/s) | Lag Time (h) |
|---|---|---|---|
| F5MTZ | 17.7 ± 1.3 | 5.02 ± 0.37 | 1.59 ± 0.09 |
| F5IPM-MTZ *,# | 29.44 ± 7.5 | 8.43 ± 2.14 | 1.61 ± 023 |
| F5PG-MTZ *,# | 26.8 ± 4.79 | 7.69 ± 1.37 | 1.47 ± 0.21 |
| F5DMSO-MTZ | 17.08 ± 4.73 | 4.82 ± 1.33 | 1.60 ± 0.44 |
| F5EtOH-MTZ | 5.68 ± 1.01 | 1.59 ± 0.28 | 2.1 ± 0.23 |
| F5TRANS-MTZ | 3.54 ± 0.79 | 0.99 ± 0.22 | 1.86 ± 0.2 |
| Viscosity (mPa·s) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rpm | F5-MTZ | F5IPM-MTZ | F5PG-MTZ | F5DMSO-MTZ | F5EtOH-MTZ | F5TRANS-MTZ | ||||||
| Upward | Downward | Upward | Downward | Upward | Downward | Upward | Downward | Upward | Downward | Upward | Downward | |
| 0.01 | 3.75 × 105 | 8.75 × 104 | 6.12 × 105 | 1.68 × 105 | 5.75 × 105 | 1.75 × 105 | 7.19 × 105 | 1.81 × 105 | 6.81 × 105 | 1.50 × 105 | 7.50 × 105 | 1.62 × 105 |
| 5 | 1.02 × 103 | 8.62 × 102 | 1.67 × 103 | 1.25 × 103 | 1.22 × 103 | 1.04 × 103 | 1.62 × 103 | 1.21 × 103 | 1.63 × 103 | 1.02 × 103 | 1.55 × 103 | 1.07 × 103 |
| 10 | 6.25 × 102 | 5.31 × 102 | 1.03 × 103 | 7.62 × 102 | 7.87 × 102 | 6.75 × 102 | 9.50 × 102 | 7.31 × 102 | 8.50 × 102 | 6.00 × 102 | 8.01 × 102 | 6.44 × 102 |
| 20 | 3.72 × 102 | 3.12 × 102 | 6.47 × 102 | 4.75 × 102 | 5.01 × 102 | 4.18 × 102 | 5.44 × 102 | 4.22 × 102 | 5.19 × 102 | 3.34 × 102 | 5.53 × 102 | 3.75 × 102 |
| 50 | 1.86 × 102 | 1.65 × 102 | 3.21 × 102 | 2.63 × 102 | 2.50 × 102 | 2.18 × 102 | 2.50 × 102 | 2.11 × 102 | 2.27 × 102 | 1.57 × 102 | 2.95 × 102 | 2.07 × 102 |
| 100 | 1.09 × 102 | 1.09 × 102 | 1.76 × 102 | 1.76 × 102 | 1.41 × 102 | 1.41 × 102 | 1.33 × 102 | 1.33 × 102 | 1.02 × 102 | 1.02 × 102 | 1.49 × 102 | 1.49 × 102 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahlizar, S.; Futaki, M.; Okada, A.; Yatomi, C.; Todo, H.; Sugibayashi, K. Combined Use of N-Palmitoyl-Glycine-Histidine Gel and Several Penetration Enhancers on the Skin Permeation and Concentration of Metronidazole. Pharmaceutics 2018, 10, 163. https://doi.org/10.3390/pharmaceutics10040163
Dahlizar S, Futaki M, Okada A, Yatomi C, Todo H, Sugibayashi K. Combined Use of N-Palmitoyl-Glycine-Histidine Gel and Several Penetration Enhancers on the Skin Permeation and Concentration of Metronidazole. Pharmaceutics. 2018; 10(4):163. https://doi.org/10.3390/pharmaceutics10040163
Chicago/Turabian StyleDahlizar, Sabrina, Mika Futaki, Akie Okada, Chihiro Yatomi, Hiroaki Todo, and Kenji Sugibayashi. 2018. "Combined Use of N-Palmitoyl-Glycine-Histidine Gel and Several Penetration Enhancers on the Skin Permeation and Concentration of Metronidazole" Pharmaceutics 10, no. 4: 163. https://doi.org/10.3390/pharmaceutics10040163
APA StyleDahlizar, S., Futaki, M., Okada, A., Yatomi, C., Todo, H., & Sugibayashi, K. (2018). Combined Use of N-Palmitoyl-Glycine-Histidine Gel and Several Penetration Enhancers on the Skin Permeation and Concentration of Metronidazole. Pharmaceutics, 10(4), 163. https://doi.org/10.3390/pharmaceutics10040163