Chitosan-Polylactide/Hyaluronic Acid Complex Microspheres as Carriers for Controlled Release of Bioactive Transforming Growth Factor-β1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CH-PLA Copolymers
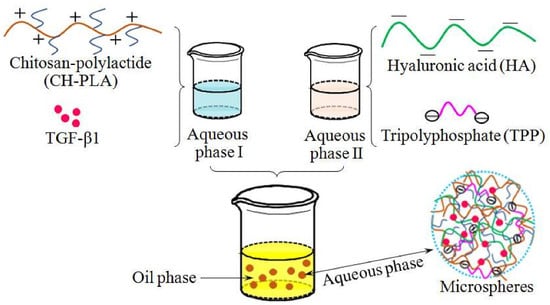
2.3. Preparation of Microspheres
2.4. Characterization
2.5. Determination of TGF-β1 Encapsulating Efficiency
2.6. Swelling Index
2.7. In Vitro Release of TGF-β1
2.8. Activity Assessment of Released TGF-β1
2.9. Statistical Analysis
3. Results and Discussions
3.1. CH-PLA Characterization
3.2. Parameters of Microspheres
3.3. Encapsulating Efficiency and Swelling Property of Microspheres
3.4. Release Profiles of Microspheres
3.5. TGF-β1 Bioactivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hunziker, E.B. Articular cartilage repair: Basic science and clinical progress. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular cartilage: From formation to tissue engineering. Biomater. Sci. 2016, 4, 734–767. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.; Jiang, C.C. Repair of articular cartilage defects: Review and perspectives. J. Formos. Med. Assoc. 2009, 108, 87–101. [Google Scholar] [CrossRef]
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue engineering and regenerative medicine: History, progress and challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Edgar, L.; McNamara, K.; Wong, T.; Tamburrini, R.; Katari, R.; Orlando, G. Heterogeneity of scaffold biomaterials in tissue engineering. Materials 2016, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Lu, S.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of biologics for cartilage repair. Adv. Drug Deliv. Rev. 2015, 84, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Hunziker, E.B. Growth-factor-induced healing of partial-thickness defects in adult articular cartilage. Osteoarthr. Cartil. 2001, 9, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M.J.; Quirk, R.A.; Howdle, S.M.; Shakesheff, K.M. Growth factor release from tissue engineering scaffolds. J. Pharm. Pharm. 2001, 53, 1427–1437. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Yuk, S.H. Polymeric protein delivery systems. Prog. Polym. Sci. 2007, 32, 669–697. [Google Scholar] [CrossRef]
- Wan, Y.; Wen, D. Preparation and characterization of porous conducting poly (dl-lactide) composite membranes. J. Membr. Sci. 2005, 246, 193–201. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Campana, R.; Skouras, A.; Bonacucina, G.; Cespi, M.; Mastrotto, F.; Baffone, W.; Casettari, L. Chitosan loaded into a hydrogel delivery system as a strategy to treat vaginal co-infection. Pharmaceutics 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Park, J.H.; Cho, Y.W.; Chung, H.; Jeong, S.Y.; Lee, E.B.; Kwon, I.C. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-β1: Implications for cartilage tissue engineering. J. Control. Release 2003, 91, 365–374. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier: Systematic examination of fabrication conditions for efficient loading and release. Colloids Surf. B Biointerfaces 2007, 59, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Gao, H.; Voronin, D.V.; Gorin, D.A.; Sukhorukov, G.B. Inorganic/organic multilayer capsule composition for improved functionality and external triggering. Adv. Mater. Interfaces 2017, 4, 1600338. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic acid decorated naringenin nanoparticles: Appraisal of chemopreventive and curative potential for lung cancer. Pharmaceutics 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Hemmati-Sadeghi, S.; Ringe, J.; Dehne, T.; Haag, R.; Sittinger, M. Hyaluronic acid influence on normal and osteoarthritic tissue-engineered cartilage. Int. J. Mol. Sci. 2018, 19, 1519. [Google Scholar] [CrossRef] [PubMed]
- Duceppe, N.; Tabrizian, M. Factors influencing the transfection efficiency of ultra low molecular weight chitosan/hyaluronic acid nanoparticles. Biomaterials 2009, 30, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.D.; Zhao, H.Q.; Wang, K.; Lv, L.L. Novel hyaluronic acid-chitosan nanoparticles as non-viral gene delivery vectors targeting osteoarthritis. Int. J. Pharm. 2011, 420, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Fuente, M.D.L.; Seijo, B.; Alonso, M.J. Bioadhesive hyaluronan-chitosan nanoparticlescan transport genes across the ocular mucosa and transfect ocular tissue. Gene Ther. 2008, 15, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Bui, V.T. Ionic conductivity and tensile properties of hydroxyethyl and hydroxypropyl chitosan membranes. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1379–1397. [Google Scholar] [CrossRef]
- Feng, H.; Dong, C.M. Synthesis and characterization of phthaloyl-chitosan-g-poly(l-lactide) using an organic catalyst. Carbohydr. Polym. 2007, 70, 258–264. [Google Scholar] [CrossRef]
- Liu, L.; Shi, A.; Guo, S.; Fang, Y.; Chen, S.; Li, J. Preparation of chitosan-g-polylactide graft copolymers via self-catalysis of phthaloylchitosan and their complexation with DNA. React. Funct. Polym. 2010, 70, 301–305. [Google Scholar] [CrossRef]
- Kim, M.S.; Ahn, S.M.; Moon, A. In vitro bioassay for transforming growth factor-β using XTT method. Arch. Pharm. Res. 2002, 25, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, J.; Xiao, B.; Zan, X.; Gao, J.; Wan, Y. N-(2-hydroxypropyl)-3-trimethylammonium chitosan-poly(ε-caprolactone) copolymers and their antibacterial activity. Carbohydr. Polym. 2011, 83, 824–830. [Google Scholar] [CrossRef]
- Yao, F.; Chen, W.; Wang, H.; Liu, H.; Yao, K.; Sun, P.; Lin, H.A. Study on cytocompatible poly(chitosan-g-l-lactic acid). Polymer 2003, 44, 6435–6441. [Google Scholar] [CrossRef]
- Cacciotti, I.; Ciocci, M.; Giovanni, E.D.; Nanni, F.; Melino, S. Hydrogen sulfide-releasing fibrous membranes: Potential patches for stimulating human stem cells proliferation and viability under oxidative stress. Int. J. Mol. Sci. 2018, 19, 2368. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G.H. Chitosan nanoparticles prepared by ionotropic gelation: An overview of recent advances. Crit. Rev. Ther. Drug Carrier Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Guo, C.; Shi, Y.; Li, L.C. Recent advances in polymeric microspheres for parenteral drug delivery-part 1. Expert Opin. Drug Deliv. 2012, 9, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohydr. Polym. 2011, 83, 1433–1445. [Google Scholar] [CrossRef]
- Almeida, J.F.; Fonseca, A.; Baptista, C.M.S.G.; Leite, E.; Gil, M.H. Immobilization of drugs for glaucoma treatment. J. Mater. Sci. Mater. Med. 2007, 18, 2309–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Yu, Y.; Wu, H. Preparation of CS/GPTMS hybrid molecularly imprinted membrane for efficient chiral resolution of phenylalanine isomers. J. Membr. Sci. 2006, 280, 876–882. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, K.E.; Kwon, I.C.; Ahn, H.J.; Lee, S.H.; Cho, H.; Kim, H.J.; Seong, S.C.; Lee, M.C. Effects of the controlled-released TGF-β1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials 2004, 25, 4163–4173. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan—A review. J. Control. Release 2006, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Peppas, L.B. Polymers in controlled drug delivery. Med. Plast. Biomater. 1997, 4, 34–44. [Google Scholar]
- Giannoni, P.; Hunziker, E.B. Release kinetics of transforming growth factor-β1 from fibrin clots. Biotechnol. Bioeng. 2003, 83, 121–123. [Google Scholar] [CrossRef] [PubMed]
- DeFail, A.J.; Chu, C.R.; Izzo, N.; Marra, K.G. Controlled release of bioactive TGF-β1 from microspheres embedded within biodegradable hydrogels. Biomaterials 2006, 27, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.A.T.C.; Brunner, G.; Walboomers, X.F.; Von Den Hoff, J.W.; Maltha, J.C.; Jansen, J.A. Release of bioactive transforming growth factor β3 from microtextured polymer surfaces in vitro and in vivo. Tissue Eng. 2002, 8, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, S.B.; Denker, A.E.; Tuan, R.S. In vitro characterization of transforming growth factor-beta1-loaded composites of biodegradable polymer and mesenchymal cells. Cells Mater. 1995, 5, 231–244. [Google Scholar]





| Copolymer Name | Feed Ratio of LA to PHCH (Molar Ratio) (a) | PLA percentage in CH-PLA (wt %) (b) | Solubility (c) | |
|---|---|---|---|---|
| DMSO | Acetic Acid (1.0%) | |||
| CH-PLA(1) | 2/1 | 23.6 (±1.51) | − | + |
| CH-PLA(2) | 4/1 | 38.7 (±1.46) | ± | + |
| CH-PLA(3) | 6/1 | 46.1 (±1.64) | ±± | ±± |
| CH-PLA(4) | 8/1 | 52.4 (±1.71) | ±± | ±± |
| Microsphere Name | Feed Ratio of HA to CH-PLA (mg/mg) | Feed Ratio of TPP to Matrix (mg/mg) | Average Size (µm) | EE (%) | SI (%) |
|---|---|---|---|---|---|
CH-I  | − | 2/1 | 3.73 (±0.41) | 29.6 (±3.4) | 69.7 (±5.1) |
| CH/HA-I(1) | 0.15/1.0 | 2/1 | 3.72 (±0.35) | 35.5 (±2.7) | 61.5 (±4.6) |
| CH/HA-I(2) | 0.3/1.0 | 2/1 | 3.74 (±0.32) | 40.7 (±2.9) | 59.1 (±3.9) |
| CH-PLA/HA-I(1) | 0.15/1.0 | 2/1 | 3.86 (±0.29) | 55.2 (±2.8) | 46.4 (±3.5) |
| CH-PLA/HA-I(2) | 0.3/1.0 | 2/1 | 4.08 (±0.25) | 61.4 (±2.5) | 44.6 (±3.1) |
CH-II  | − | 4/1 | 3.16 (±0.27) | 37.1 (±3.3) | 61.2 (±4.8) |
| CH/HA-II(a) | 0.15/1.0 | 4/1 | 3.46 (±0.31) | 46.8 (±3.1) | 53.4 (±4.3) |
| CH/HA-II(b) | 0.3/1.0 | 4/1 | 3.63 (±0.28) | 51.6 (±3.6) | 48.5 (±3.6) |
| CH-PLA/HA-II(a) | 0.15/1.0 | 4/1 | 3.83 (±0.24) | 75.3 (±3.2) | 32.3 (±3.4) |
| CH-PLA/HA-II(b) | 0.3/1.0 | 4/1 | 4.16 (±0.28) | 81.9 (±3.8) | 30.2 (±3.2) |
 ,
,  ) Different sample sets: set-one (
) Different sample sets: set-one (  ) and set-two (
) and set-two (  ).
).© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, Q.; Liu, J.; Li, J.; Wan, Y.; Wu, J. Chitosan-Polylactide/Hyaluronic Acid Complex Microspheres as Carriers for Controlled Release of Bioactive Transforming Growth Factor-β1. Pharmaceutics 2018, 10, 239. https://doi.org/10.3390/pharmaceutics10040239
Min Q, Liu J, Li J, Wan Y, Wu J. Chitosan-Polylactide/Hyaluronic Acid Complex Microspheres as Carriers for Controlled Release of Bioactive Transforming Growth Factor-β1. Pharmaceutics. 2018; 10(4):239. https://doi.org/10.3390/pharmaceutics10040239
Chicago/Turabian StyleMin, Qing, Jiaoyan Liu, Jing Li, Ying Wan, and Jiliang Wu. 2018. "Chitosan-Polylactide/Hyaluronic Acid Complex Microspheres as Carriers for Controlled Release of Bioactive Transforming Growth Factor-β1" Pharmaceutics 10, no. 4: 239. https://doi.org/10.3390/pharmaceutics10040239
APA StyleMin, Q., Liu, J., Li, J., Wan, Y., & Wu, J. (2018). Chitosan-Polylactide/Hyaluronic Acid Complex Microspheres as Carriers for Controlled Release of Bioactive Transforming Growth Factor-β1. Pharmaceutics, 10(4), 239. https://doi.org/10.3390/pharmaceutics10040239