Dealing with Skin and Blood-Brain Barriers: The Unconventional Challenges of Mesoporous Silica Nanoparticles
Abstract
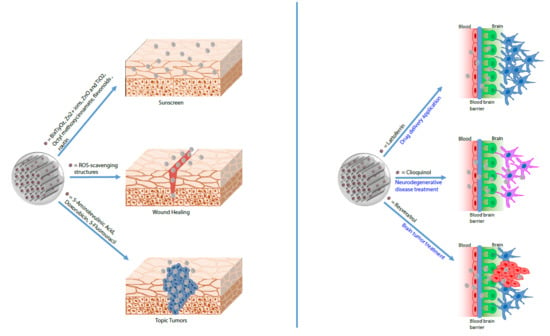
:1. Introduction
2. Drug Delivery Systems (DDSs)
3. MSNs in Topical Drug Delivery
4. MSNs in Cosmetics
5. MSNs in the Topical Treatment of Cancer
6. Drug Delivery as a Potential Approach to Cross the Blood-Brain Barrier (BBB)
7. MSNs as Drug Delivery Systems Targeting the BBB
8. MSNs-Based Therapy in Alzheimer’s Disease
9. MSNs-Based Therapy to Cure Brain Tumors
10. Neurodegenerative Disease and MSNs Therapy
11. Conclusions
Funding
Conflicts of Interest
References
- Ma, Y.; Mou, Q.B.; Zhu, X.Y.; Yan, D.Y. Small molecule nanodrugs for cancer therapy. Mater. Today Chem. 2017, 4, 26–39. [Google Scholar] [CrossRef]
- Sayed, E.; Haj-Ahmad, R.; Ruparelia, K.; Arshad, M.S.; Chang, M.W.; Ahmad, Z. Porous Inorganic Drug Delivery Systems—A Review. AAPS PharmSciTech 2017, 18, 1507–1525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.B.D.; Corgosinho, L.D.; Faria, J.A.Q.A.; dos Santos, V.M.; Resende, J.M.; Leal, A.S.; Gomes, D.A.; de Sousa, E.M.B. Multifunctional mesoporous silica nanoparticles for cancer-targeted, controlled drug delivery and imaging. Micropor. Mesopor. Mater. 2017, 242, 271–283. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Ramila, A.; del Real, R.P.; Perez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.P.; Apolinario, L.M.; Favaro, W.J.; Paula, A.J.; Duran, N. Doxorubicin-Functionalized Silica Nanoparticles Incorporated into a Thermoreversible Hydrogel and Intraperitoneally Administered Result in High Prostate Antitumor Activity and Reduced Cardiotoxicity of Doxorubicin. ACS Biomater. Sci. Eng. 2016, 2, 1190–1199. [Google Scholar] [CrossRef]
- Cavallaro, G.; Pierro, P.; Palumbo, F.S.; Testa, F.; Pasqua, L.; Aiello, R. Drug delivery devices based on mesoporous silicate. Drug Deliv. 2004, 11, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Carino, I.S.; Pasqua, L.; Testa, F.; Aiello, R.; Puoci, F.; Iemma, F.; Picci, N. Silica-based mesoporous materials as drug delivery system for methotrexate release. Drug Deliv. 2007, 14, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Salcedo, S.; Vallet-Regi, M.; Shahin, S.A.; Glackin, C.A.; Zink, J.I. Mesoporous core-shell silica nanoparticles with anti-fouling properties for ovarian cancer therapy. Chem. Eng. J. 2018, 340, 114–124. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Zhang, A.Q.; Hu, J.J.; He, F.; Zeng, X.; Zhang, X.Z. Multifunctional Peptide-Amphiphile End-Capped Mesoporous Silica Nanoparticles for Tumor Targeting Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, L.; Testa, F.; Aiello, R.; Cundari, S.; Nagy, J.B. Preparation of bifunctional hybrid mesoporous silica potentially useful for drug targeting. Microporous Microporous Mater. 2007, 103, 166–173. [Google Scholar] [CrossRef]
- Pasqua, L.; Leggio, A.; Sisci, D.; Ando, S.; Morelli, C. Mesoporous Silica Nanoparticles in Cancer Therapy: Relevance of the Targeting Function. Mini Rev. Med. Chem. 2016, 16, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Lopez, V.; Villegas, M.R.; Rodriguez, V.; Villaverde, G.; Lozano, D.; Baeza, A.; Vallet-Regi, M. Janus Mesoporous Silica Nanoparticles for Dual Targeting of Tumor Cells and Mitochondria. ACS Appl. Mater. Interfaces 2017, 9, 26697–26706. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; de la Torre, P.; Manzano, M.; Cabanas, M.V.; Flores, A.I.; Vallet-Regi, M. Decidua-derived mesenchymal stem cells as carriers of mesoporous silica nanoparticles. In vitro and in vivo evaluation on mammary tumors. Acta Biomater. 2016, 33, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wang, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Nanomedicine 2012, 7, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, E. Value of phagocyte function screening for immunotoxicity of nanoparticles in vivo. Int. J. Nanomed. 2015, 10, 3761–3778. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Bergman, L.; Kankaanpaa, P.; Tiitta, S.; Duchanoy, A.; Li, L.; Heino, J.; Linden, M. Intracellular Degradation of Multilabeled Poly(Ethylene imine)-Mesoporous Silica-Silica Nanoparticles: Implications for Drug Release. Mol. Pharm. 2013, 10, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.Q.; Fu, A.; Shi, W.; Yeo, D.; Luo, K.Q.; Ow, H.; Xu, C.J. Tracking mesenchymal stem cell tumor-homing using fluorescent silica nanoparticles. J. Mater. Chem. B 2015, 3, 1245–1253. [Google Scholar] [CrossRef]
- Nakamura, T.; Sugihara, F.; Matsushita, H.; Yoshioka, Y.; Mizukami, S.; Kikuchi, K. Mesoporous silica nanoparticles for F-19 magnetic resonance imaging, fluorescence imaging, and drug delivery. Chem. Sci. 2015, 6, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, M.; Zhao, J.L.X.J. Recent development of silica nanoparticles as delivery vectors for cancer imaging and therapy. Nanomed. Nanotechnol. 2014, 10, 297–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.S.; Haynes, C.L. Impacts of Mesoporous Silica Nanoparticle Size, Pore Ordering, and Pore Integrity on Hemolytic Activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gonen, M.; Kalaigian, H.; Schoder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.Y.; Ting, X.Z.L.; Zhu, J.J. The Research Progress of Targeted Drug Delivery Systems. IOP Conf. Ser. Mater. Sci. 2017, 207. [Google Scholar] [CrossRef]
- Morelli, C.; Maris, P.; Sisci, D.; Perrotta, E.; Brunelli, E.; Perrotta, I.; Panno, M.L.; Tagarelli, A.; Versace, C.; Casula, M.F.; et al. PEG-templated mesoporous silica nanoparticles exclusively target cancer cells. Nanoscale 2011, 3, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Ceresa, C.; Nicolini, G.; Rigolio, R.; Bossi, M.; Pasqua, L.; Cavaletti, G. Functionalized mesoporous silica nanoparticles: A possible strategy to target cancer cells reducing peripheral nervous system uptake. Curr. Med. Chem. 2013, 20, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.Z.; Ilic, N.; Dokić, V.; Petrovic, R.; Janackovic, D.O.E. Mesoporous Silica and Organosilica Nanomaterials as UV-Blocking Agents. ACS Appl. Mater. Interfaces 2018, 10, 20231–20236. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Luo, L.; Liang, S.; Long, M.; Xu, H. Amino-functionalized mesoporous silica nanoparticles as efficient carriers for anticancer drug delivery. J. Biomater. Appl. 2017, 32, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, C.; Sousa, J.; Pais, A. Overcoming the skin permeation barrier: Challenges and opportunities. Curr. Pharm. Des. 2015, 21, 2698–2712. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Srivastava, S.; Singh, D.; Saraf, S.; Saraf, S.; Singh, M.R. Perspectives of Lipid-Based Drug Carrier Systems for Transdermal Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 331–367. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Oliaro-Bosso, S.; Zonari, D.; Zattoni, A.; Ugazio, E. Mesoporous silica nanoparticles as a promising skin delivery system for methotrexate. Int. J. Pharm. 2017, 530, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Zaccariello, G.; Back, M.; Zanello, M.; Canton, P.; Cattaruzza, E.; Riello, P.; Alimonti, A.; Benedetti, A. Formation and Controlled Growth of Bismuth Titanate Phases into Mesoporous Silica Nanoparticles: An Efficient Self-Sealing Nanosystem for UV Filtering in Cosmetic Formulation. ACS Appl. Mater. Interfaces 2017, 9, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Hatahet, T.; Morille, M.; Hommoss, A.; Devoisselle, J.M.; Muller, R.H.; Begu, S. Quercetin topical application, from conventional dosage forms to nanodosage forms. Eur. J. Pharm. Biopharm. 2016, 108, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Larese Filon, F.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Knorr, F.; Richter, H.; Jung, S.; Meinke, M.C.; Ruhl, E.; Alexiev, U.; Calderon, M.; Patzelt, A. Hair follicles as a target structure for nanoparticles. J. Innov. Opt. Health Sci. 2015, 8. [Google Scholar] [CrossRef]
- Sahle, F.F.; Giulbudagian, M.; Bergueiro, J.; Lademann, J.; Calderon, M. Dendritic polyglycerol and N-isopropylacrylamide based thermoresponsive nanogels as smart carriers for controlled delivery of drugs through the hair follicle. Nanoscale 2017, 9, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pang, K.Y.; Ng, T.W.; Leung, P.C.; Zhang, C.F.; Leung, K.C.; Jin, L. Cellular Interactions and Formation of an Epithelial “Nanocoating-Like Barrier” with Mesoporous Silica Nanoparticles. Nanomaterials 2016, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Mostaghaci, B.; Sitti, M. Recent Advances in Skin Penetration Enhancers for Transdermal Gene and Drug Delivery. Curr. Gene Ther. 2017, 17, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mebert, A.M.; Baglole, C.J.; Desimone, M.F.; Maysinger, D. Nanoengineered silica: Properties, applications and toxicity. Food Chem. Toxicol. 2017, 109, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.S.; Lee, Y.C.; Kuo, Y.C.; Lin, C.C. Development of Octyl Methoxy Cinnamates (OMC)/Silicon Dioxide (SiO(2)) Nanoparticles by Sol-Gel Emulsion Method. Nanomaterials 2017, 7, 434. [Google Scholar] [CrossRef] [PubMed]
- Ugazio, E.; Gastaldi, L.; Brunella, V.; Scalarone, D.; Jadhav, S.A.; Oliaro-Bosso, S.; Zonari, D.; Berlier, G.; Miletto, I.; Sapino, S. Thermoresponsive mesoporous silica nanoparticles as a carrier for skin delivery of quercetin. Int. J. Pharm. 2016, 511, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, H.; Choi, Y.; Lee, D.S.; Kim, J.; Yi, G.R. Colloidal Mesoporous Silica Nanoparticles as Strong Adhesives for Hydrogels and Biological Tissues. ACS Appl. Mater. Interfaces 2017, 9, 31469–31477. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous silica as topical nanocarriers for quercetin: Characterization and in vitro studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ebabe Elle, R.; Rahmani, S.; Lauret, C.; Morena, M.; Bidel, L.P.; Boulahtouf, A.; Balaguer, P.; Cristol, J.P.; Durand, J.O.; Charnay, C.; et al. Functionalized Mesoporous Silica Nanoparticle with Antioxidants as a New Carrier That Generates Lower Oxidative Stress Impact on Cells. Mol. Pharm. 2016, 13, 2647–2660. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Yang, F.; Liu, J.; Hu, H.; Du, X.; Hanagata, N.; Zhao, S.; Zhu, Y. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment. Biomater. Sci. 2018, 6, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, Q.; Zhao, Y. Targeted delivery of 5-aminolevulinic acid by multifunctional hollow mesoporous silica nanoparticles for photodynamic skin cancer therapy. ACS Appl. Mater. Interfaces 2015, 7, 10671–10676. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Carmona, M.; Baeza, A.; Rodriguez-Milla, M.A.; Garcia-Castro, J.; Vallet-Regi, M. Mesoporous silica nanoparticles grafted with a light-responsive protein shell for highly cytotoxic antitumoral therapy. J. Mater. Chem. B 2015, 3, 5746–5752. [Google Scholar] [CrossRef] [Green Version]
- Anirudhan, T.S.; Nair, A.S. Temperature and ultrasound sensitive gatekeepers for the controlled release of chemotherapeutic drugs from mesoporous silica nanoparticles. J. Mater. Chem. B 2018, 6, 428–439. [Google Scholar] [CrossRef]
- Singh, P.; Singh, H.; Castro-Aceituno, V.; Ahn, S.; Kim, Y.J.; Farh, M.E.; Yang, D.C. Engineering of mesoporous silica nanoparticles for release of ginsenoside CK and Rh2 to enhance their anticancer and anti-inflammatory efficacy: In vitro studies. J. Nanopart. Res. 2017, 19. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Watson, C.; Murdaugh, K.M.; Darrah, T.H.; Pyrgiotakis, G.; Elder, A.; Brain, J.D.; Demokritou, P. Engineering safer-by-design, transparent, silica-coated ZnO nanorods with reduced DNA damage potential. Environ. Sci. Nano 2014, 1, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, S.H.; McFadden, P.D.; Loy, D.A. New Hybrid Organic/Inorganic Polysilsesquioxane-Silica Particles as Sunscreens. ACS Appl. Mater. Interfaces 2016, 8, 3160–3174. [Google Scholar] [CrossRef] [PubMed]
- Bagde, A.; Mondal, A.; Singh, M. Drug delivery strategies for chemoprevention of UVB-induced skin cancer: A review. Photodermatol. Photoimmunol. Photomed. 2018, 34, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wong, C.H.; Ng, T.W.; Zhang, C.F.; Leung, K.C.; Jin, L. The spherical nanoparticle-encapsulated chlorhexidine enhances anti-biofilm efficiency through an effective releasing mode and close microbial interactions. Int. J. Nanomed. 2016, 11, 2471–2480. [Google Scholar] [CrossRef]
- Lungare, S.; Hallam, K.; Badhan, R.K. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016, 513, 280–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, K.K. Nanobiotechnology-based strategies for crossing the blood-brain barrier. Nanomedicine 2012, 7, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards Improvements for Penetrating the Blood-Brain Barrier-Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Baghirov, H.; Karaman, D.; Viitala, T.; Duchanoy, A.; Lou, Y.R.; Mamaeva, V.; Pryazhnikov, E.; Khiroug, L.; de Lange Davies, C.; Sahlgren, C.; et al. Feasibility Study of the Permeability and Uptake of Mesoporous Silica Nanoparticles across the Blood-Brain Barrier. PLoS ONE 2016, 11, e0160705. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praca, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sui, B.; Sun, J. Blood-brain barrier dysfunction induced by silica NPs in vitro and in vivo: Involvement of oxidative stress and Rho-kinase/JNK signaling pathways. Biomaterials 2017, 121, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ke, W.; Han, L.; Liu, Y.; Shao, K.; Jiang, C.; Pei, Y. Lactoferrin-modified nanoparticles could mediate efficient gene delivery to the brain in vivo. Brain Res. Bull. 2010, 81, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, D.; Li, L.; Xu, J.; Dutta, P.; Lin, Y. In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier. ACS Appl. Mater. Interfaces 2017, 9, 20410–20416. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Li, M.; Wu, L.; Chen, C.; Qu, X. Mesoporous silica nanoparticle-based H2O2 responsive controlled-release system used for Alzheimer’s disease treatment. Adv. Healthc. Mater. 2012, 1, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yin, T.; Liu, Y.; Sun, J.; Zhou, Y.; Liu, J. Gold nanoparticle-capped mesoporous silica-based H2O2-responsive controlled release system for Alzheimer’s disease treatment. Acta Biomater. 2016, 46, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, M.; Rashidi, L.; Ganji, F. Mesoporous silica nanoparticles for efficient rivastigmine hydrogen tartrate delivery into SY5Y cells. Drug Dev. Ind. Pharm. 2017, 43, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cao, B.; Snyder, N.R.; Woeppel, K.M.; Eles, J.R.; Cui, X.T. ROS responsive resveratrol delivery from LDLR peptide conjugated PLA-coated mesoporous silica nanoparticles across the blood-brain barrier. J. Nanobiotechnol. 2018, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- You, Y.Y.; Yang, L.Y.; He, L.Z.; Chen, T.F. Tailored mesoporous silica nanosystem with enhanced permeability of the blood-brain barrier to antagonize glioblastoma. J. Mater. Chem. B 2016, 4, 5980–5990. [Google Scholar] [CrossRef]
- Mo, J.; He, L.; Ma, B.; Chen, T. Tailoring Particle Size of Mesoporous Silica Nanosystem To Antagonize Glioblastoma and Overcome Blood-Brain Barrier. ACS Appl. Mater. Interfaces 2016, 8, 6811–6825. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Ali, O.A.; Anitua, E.; Pedraz, J.L.; Emerich, D.F. Biomaterial-based technologies for brain anti-cancer therapeutics and imaging. Biochim. Biophys. Acta 2010, 1806, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D.D. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, U.; Sommerfeld, P.; Ulrich, S.; Sabel, B.A. Nanoparticle technology for delivery of drugs across the blood-brain barrier. J. Pharm. Sci. 1998, 87, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Sakaeda, T.; Sugawara, T.; Hirano, K.; Stella, V.J. A novel chemical delivery system for brain targeting. Adv. Drug Deliv. Rev. 1999, 36, 255–275. [Google Scholar] [CrossRef]
- Gidwani, M.; Singh, A.V. Nanoparticle enabled drug delivery across the blood brain barrier: In vivo and in vitro models, opportunities and challenges. Curr. Pharm. Biotechnol. 2014, 14, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ke, W.; Liu, Y.; Jiang, C.; Pei, Y. The use of lactoferrin as a ligand for targeting the polyamidoamine-based gene delivery system to the brain. Biomaterials 2008, 29, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Fillebeen, C.; Descamps, L.; Dehouck, M.P.; Fenart, L.; Benaissa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Jia, Q.; Huwel, S.; Xia, R.; Liu, T.; Gao, F.; Galla, H.J.; Gao, M. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano 2012, 6, 3304–3310. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Fujioka, K.; Inoue, Y.; Kanaya, F.; Manome, Y.; Yamamoto, K. Cell-based in vitro blood-brain barrier model can rapidly evaluate nanoparticles’ brain permeability in association with particle size and surface modification. Int. J. Mol. Sci. 2014, 15, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Maeda, J.; Higuchi, M.; Inoue, K.; Akita, H.; Harashima, H.; Suhara, T. Pharmacokinetics and brain uptake of lactoferrin in rats. Life Sci. 2006, 78, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Y.; Chen, W.J.; Lee, W.Y.; Lo, S.T.; Lee, T.W.; Lo, J.M. In vitro and in vivo evaluation of lactoferrin-conjugated liposomes as a novel carrier to improve the brain delivery. Int. J. Mol. Sci. 2013, 14, 2862–2874. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, R.B.; Farias, G.; Morales, I.; Navarrete, L. The revitalized tau hypothesis on Alzheimer’s disease. Arch. Med. Res. 2010, 41, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wen, H.; Lu, W.L.; Du, J.; Guo, J.; Tian, W.; Men, Y.; Zhang, Y.; Li, R.J.; Yang, T.Y.; et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J. Controll. Release 2010, 141, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Vredenburgh, J.J.; Desjardins, A.; Reardon, D.A.; Peters, K.B.; Herndon, J.E., 2nd; Marcello, J.; Kirkpatrick, J.P.; Sampson, J.H.; Bailey, L.; Threatt, S.; et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin. Cancer Res. 2011, 17, 4119–4124. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Jiang, M.; Jiang, D.; Feng, X.; Yao, J.; Song, Q.; Chen, H.; Gao, X.; Chen, J. Enhancing Glioblastoma-Specific Penetration by Functionalization of Nanoparticles with an Iron-Mimic Peptide Targeting Transferrin/Transferrin Receptor Complex. Mol. Pharm. 2015, 12, 2947–2961. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chiang, C.F.; Wu, S.K.; Chen, L.F.; Hsieh, W.Y.; Lin, W.L. Targeting microbubbles-carrying TGFbeta1 inhibitor combined with ultrasound sonication induce BBB/BTB disruption to enhance nanomedicine treatment for brain tumors. J. Controll. Release. 2015, 211, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Solanki, I.; Parihar, P.; Mansuri, M.L.; Parihar, M.S. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 2015, 6, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B. Blueberries and neuronal aging. Gerontology 2012, 58, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Tinarelli, C.; Schulz, R.J.; Polidori, M.C. Nutraceuticals in cognitive impairment and Alzheimer’s disease. Front. Pharmacol. 2014, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Macready, A.L.; Kennedy, O.B.; Ellis, J.A.; Williams, C.M.; Spencer, J.P.; Butler, L.T. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009, 4, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, E.P. A berry thought-provoking idea: The potential role of plant polyphenols in the treatment of age-related cognitive disorders. Br. J. Nutr. 2012, 108, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.B.; Ding, E.L.; Dixon, R.; Pasinetti, G.M.; Villarreal, F. The science of cocoa flavanols: Bioavailability, emerging evidence, and proposed mechanisms. Adv. Nutr. 2014, 5, 547–549. [Google Scholar] [CrossRef] [PubMed]


| Application | Structure | Features | References |
|---|---|---|---|
| Cosmetics | Bismuth titanates (BixTiyOz) NPs embedded into MSNs. | Inorganic sunscreen UV filters. | [39] |
| MSNs and periodic mesoporous organosilica NPs functionalized with a chelating ligand and Zn2+ ions and containing bridging benzene and ethane moieties. | Photostable and safe sunscreen UV filters. | [33] | |
| Octyl methoxy cinnamates molecules encapsulated into Hollow Silica NPs. | Sunscreen UV filters. | [47] | |
| MSNs functionalized with N-isopropylacrylamide a thermoresponsive copolymer, 3-(methacryloxypropyl)trimethoxysilane inside the mesopores and loaded with quercetin. | Topical carriers for quercetin, antioxidant and labile active ingredients of dermocosmetic interest. | [48] | |
| Biomedical | Ceria nanocrystals immobilized onto the surface of MSNs | Tissue adhesive capability and in vivo ROS-scavenging activity | [49] |
| MSNs and fluorescent MSNs. | Potential MSN-based anti-infective and anti-inflammatory agents in topical applications for effective oral healthcare. | [44] | |
| Spherical Colloidal MSNs with ordered mesopores. | Strong adhesives for hydrogels and biological tissues. | [50] | |
| MSNs and Methotrexate complex. | Dermal delivery of Methotrexate for the treatment of skin diseases. | [38] | |
| MSNs loaded with quercetin. | Potential topical carrier to load flavonoids derivatives. | [51] | |
| MSNs covalently coated with antioxidant molecules, caffeic acid or rutin. | New carrier with antioxidant properties. | [52] | |
| Cancer | Transcutaneous delivery plat form consisting of Doxorubicin hydrochloride and indocyanine green conjugated with silica NPs loaded on microneedle patches. | Treating superficial tumors using a combination of chemotherapy and photothermal therapy. | [53] |
| Multifunctional hollow MSNs containing PEG and folic acid targeting ligand and loaded with 5-Aminolevulinic Acid. | Potential in photodynamic skin cancer therapy. | [54] | |
| MSNs loaded with Doxorubicin and decorated with a biocompatible protein shell cleavable by light irradiation. | Treatment of exposed tumors that affect the skin, oesophagus, and stomach and are easily accessible for light irradiation. | [55] | |
| (tetrahydropyranyl methacrylate co-amino ethyl methacrylate)-grafted-mesoporous silica nanoparticles loaded with 5-flurouracil. | Potential applicability in site selective transdermal delivery of chemotherapeutic drugs. | [56] | |
| MSNs loaded with two ginsenosides: ginsenoside compound K and Rh2. | Potential candidate to load ginsenosides with anti-cancer and anti-inflammatory efficacy. | [57] |
| Application | Structure | Features | References |
|---|---|---|---|
| Drug Delivery | MSNs surface coated with Polyamidoamine (PAMAM), polyethylene glycol (PEG) and lactoferrin (Lf). | Lactoferrin-modified NPs, a ligand for brain-targeting drug delivery systems. | [68] |
| MSNs surface modified with PEG (PSi NPs) and conjugated with Lf. | Brain drug delivery probe by covalently binding Lf to PSi NPs to achieve receptor-mediated delivery of NPs across the BBB | [69] | |
| Neurodegenerative Diseases | MSNs surface coated with a suitable derivative of the arylboronic acids, 3-carboxyphenylboronic acid (MSN-BA). MSN loaded with the dye rhodamine B and capped with human IgG. | MSN based H2O2 responsive controlled-release system used for Alzheimer’s Disease Treatment | [70] |
| Gold nanoparticle-capped mesoporous silica (MSN-AuNPs): a H2O2-responsive controlled release system for targeted delivery of the metal chelator clioquinol (CQ). | Inhibition of the amyloid-β aggregation and of formation of neurotoxic ROS in the Alzheimer’s disease treatment. | [71] | |
| MSNs loaded with Rivastigmine hydrogen tartrate, a carbamate-derived reversible cholinesterase inhibitor that is selective for the central nervous system. | NPs used to treat confusion (dementia) related to Alzheimer’s disease and Parkinson’s disease. | [72] | |
| Polylactic acid (PLA)-coated MSNs (MSNPs), conjugated with a ligand peptide of the low-density lipoprotein receptor (LDLR) and loaded with resveratrol. | A Resveratrol delivery system for the treatment of various central nervous system disorders associated with oxidative stress. | [73] | |
| MSNs loaded with the phytochemicals curcumin and chrysin. | Nose-to-brain delivery system for the treatment of various central nervous system disorders. | [62] | |
| Brain Tumor | Nanosystem modified by RGD (arginine–glycine–aspartate) peptide useful as a carrier of anticancer agents, by using a novel organic selenium compound BSeC as a potential chemotherapeutic agent. | New strategy for the rational design of a tailored nanomedicine with enhanced BBB permeability to treat human brain glioma. | [74] |
| MSNs conjugated with cRGD peptide to enhance its cancer targeting effect, and loaded with the antineoplastic drug doxorubicin. | The functionalized nanosystem selectively recognizes glioma cells, inducing apoptosis by triggering ROS overproduction. | [75] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigro, A.; Pellegrino, M.; Greco, M.; Comandè, A.; Sisci, D.; Pasqua, L.; Leggio, A.; Morelli, C. Dealing with Skin and Blood-Brain Barriers: The Unconventional Challenges of Mesoporous Silica Nanoparticles. Pharmaceutics 2018, 10, 250. https://doi.org/10.3390/pharmaceutics10040250
Nigro A, Pellegrino M, Greco M, Comandè A, Sisci D, Pasqua L, Leggio A, Morelli C. Dealing with Skin and Blood-Brain Barriers: The Unconventional Challenges of Mesoporous Silica Nanoparticles. Pharmaceutics. 2018; 10(4):250. https://doi.org/10.3390/pharmaceutics10040250
Chicago/Turabian StyleNigro, Alessandra, Michele Pellegrino, Marianna Greco, Alessandra Comandè, Diego Sisci, Luigi Pasqua, Antonella Leggio, and Catia Morelli. 2018. "Dealing with Skin and Blood-Brain Barriers: The Unconventional Challenges of Mesoporous Silica Nanoparticles" Pharmaceutics 10, no. 4: 250. https://doi.org/10.3390/pharmaceutics10040250
APA StyleNigro, A., Pellegrino, M., Greco, M., Comandè, A., Sisci, D., Pasqua, L., Leggio, A., & Morelli, C. (2018). Dealing with Skin and Blood-Brain Barriers: The Unconventional Challenges of Mesoporous Silica Nanoparticles. Pharmaceutics, 10(4), 250. https://doi.org/10.3390/pharmaceutics10040250