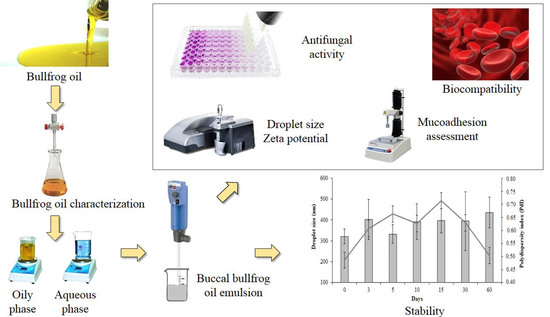
Buccal Bullfrog (Rana catesbeiana Shaw) Oil Emulsion: A Mucoadhesive System Intended for Treatment of Oral Candidiasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Biological
2.2. Methods
2.2.1. Physicochemical Characterization of Bullfrog Oil
2.2.2. Production of Buccal Emulsified System Containing Bullfrog Oil
2.2.3. Buccal Bullfrog Oil Characterization
2.2.4. Macroscopic Aspects
2.2.5. pH and Conductivity Evaluation
2.2.6. Droplet Size Distribution and Zeta Potential Analysis
2.2.7. Viscosity Measurements
2.2.8. In Vitro Mucoadhesive Studies
Mucoadhesive Performance
Interaction between the Emulsion and the Mucin
2.2.9. Antimicrobial Activity of Bullfrog Oil and Buccal Bullfrog Oil Emulsion
Inocula Preparation
Fungal Minimal Inhibitory Concentration (MIC)—Broth Microdilution Assay
2.2.10. In vitro Biocompatibility Assay—Hemolysis in Total Blood
2.3. Statistical Analyses
3. Results
3.1. Bullfrog Oil (Rana catesbeiana Shaw) Physicochemical Characterization
3.2. Production of Buccal Bullfrog Oil Emulsion (BBE)
3.3. Evaluation of Mucoadhesive Properties of the Buccal Bullfrog Oil Emulsion
3.4. Antifungal Activity of Buccal Bullfrog Oil Emulsion
3.5. In Vitro Biocompatibility Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santosh, A.B.R.; Reddy, B.V.R. Oral mucosal infections. Dent. Clin. N. Am. 2017, 61, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P.; Keung, W.L.; Jin, L. Oral mucosal fungal infections. Periodontol 2000 2009, 49, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Schuster, G.S. Oral flora and pathogenic organisms. Infect. Dis. Clin. N. Am. 1999, 13, 757–774. [Google Scholar] [CrossRef]
- da Silva-Rocha, W.P.; Lemos, V.L.; Svidizisnki, T.I.; Milan, E.P.; Chaves, G.M. Candida species distribution, genotyping and virulence factors of candida albicans isolated from the oral cavity of kidney transplant recipients of two geographic regions of brazil. BMC Oral Health 2014, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Lewis, M. Pathogenesis and treatment of oral candidosis. J. Oral Microbiol. 2011, 3, 5771. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical appearance of oral candida infection and therapeutic strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef] [PubMed]
- Chaves, G.M.; Diniz, M.G.; da Silva-Rocha, W.P.; de Souza, L.B.; Gondim, L.A.; Ferreira, M.A.; Svidzinski, T.I.; Milan, E.P. Species distribution and virulence factors of candida spp. Isolated from the oral cavity of kidney transplant recipients in brazil. Mycopathologia 2013, 175, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cuesta, C.; Sarrion-Pérez, M.G.; Bagán, J.V. Current treatment of oral candidiasis: A literature review. J. Clin. Exp. Dent. 2014, 6, e576–e582. [Google Scholar] [CrossRef] [PubMed]
- Valle-Castillo, G.; Blanc, S.L.; Sotomayor, C.E.; Azcurra, A.I. Study of virulence factor of candida species in oral lesions and its association with potentially malignant and malignant lesions. Arch. Oral Biol. 2018, 91, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Giannini, M.J.S.M. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Milillo, L.; Marinosci, F.; Lo Muzio, L.; Serpico, R.; Antonaci, S. Altered expression of integrins and basement membrane proteins in malignant and pre-malignant lesions of oral mucosa. J. Boil. Regul. Homeost. Agents 2001, 15, 375–380. [Google Scholar]
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Sawant, B.; Khan, T. Recent advances in delivery of antifungal agents for therapeutic management of candidiasis. Biomed. Pharmacother. 2017, 96, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, D.; Lauritano, D.; Minervini, G.; Paparella, R.; Petruzzi, M.; Romano, A.; Candotto, V.; Lucchese, A. Management of denture stomatitis: A narrative review. J. Boil. Regul. Homeost. Agents 2018, 32, 113–116. [Google Scholar]
- Chinsembu, K.C. Plants and other natural products used in the management of oral infections and improvement of oral health. Acta Trop. 2016, 154, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Alencar, E.N.; Xavier-Junior, F.H.; Morais, A.R.V.; Dantas, T.R.F.; Dantas-Santos, N.; Verissimo, L.M.; Rehder, V.L.G.; Chaves, G.M.; Oliveira, A.G.; Egito, E.S.T. Chemical characterization and antimicrobial activity evaluation of natural oil nanostructured emulsions. J. Nanosci. Nanotechnol. 2015, 15, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Amaral-Machado, L.; Xavier-Junior, F.H.; Rutckeviski, R.; Morais, A.R.; Alencar, E.N.; Dantas, T.R.; Cruz, A.K.; Genre, J.; da Silva-Junior, A.A.; Pedrosa, M.F.; et al. New trends on antineoplastic therapy research: Bullfrog (rana catesbeiana shaw) oil nanostructured systems. Molecules 2016, 21, 585. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.N.; Amaral-Machado, L.S.; Alencar, E.N.; Marcelino, H.R.; Genre, J.; Silva-Rocha, W.P.; Gondim, A.D.; Chaves, G.M.; Fernandes-Pedrosa, M.F.; Egito, E.S.T. Getting the jump on the development of bullfrog oil microemulsions: A nanocarrier for amphotericin b intended for antifungal treatment. AAPS PharmSciTech 2018, 19, 2585–2597. [Google Scholar] [CrossRef] [PubMed]
- Rutckeviski, R.; Xavier-Junior, F.H.; Morais, A.R.V.; Amaral-Machado, L.; Alencar, E.N.; Genre, J.; Araujo, A.A.S.; Egito, E.S.T. Therapeutic bullfrog oil-based nanoemulsion for oral application: Development, characterization and stability. Acta Pharm. 2018, in press. [Google Scholar]
- Griffin, W.C. Classification of surface-active agents by “hlb”. J. Soc. Cos. Chem. 1949, 1, 311–326. [Google Scholar]
- Morais, A.R.V.; Alencar, E.N.; Xavier-Junior, F.H.; Oliveira, C.M.; Marcelino, H.R.; Barratt, G.; Fessi, H.; Egito, E.S.T.; Elaissari, A. Freeze-drying of emulsified systems: A review. Int. J. Pharm. 2016, 503, 102–114. [Google Scholar] [CrossRef] [PubMed]
- US Pharmacopeial Convention. US Pharmacopoeia National Formulary; US Pharmacopeial Convention: Rockville, MD, USA, 2012; Volume USP35 NF30. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS: Urbana, IL, USA, 1989. [Google Scholar]
- Fernandez, P.; André, V.; Rieger, J.; Kühnle, A. Nano-emulsion formation by emulsion phase inversion. Colloids Surf. A Physicochem. Eng. Asp. 2004, 251, 53–58. [Google Scholar] [CrossRef]
- Zatta, K.C.; Frank, L.A.; Reolon, L.A.; Amaral-Machado, L.; Egito, E.S.T.; Gremião, M.P.D.; Pohlmann, A.R.; Guterres, S.S. An inhalable powder formulation based on micro-and nanoparticles containing 5-fluorouracil for the treatment of metastatic melanoma. Nanomaterials 2018, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Santos-Chaves, P.; Ourique, A.F.; Frank, L.A.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Carvedilol-loaded nanocapsules: Mucoadhesive properties and permeability across the sublingual mucosa. Eur. J. Pharm. Biopharm. 2017, 114, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; p. 46. [Google Scholar]
- Gutiérrez, L.F.; Quiñones-Segura, Y.; Sanchez-Reinoso, Z.; Díaz, D.L.; Abril, J.I. Physicochemical properties of oils extracted from γ-irradiated sacha inchi (Plukenetia volubilis L.) seeds. Food Chem. 2017, 237, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F. Healthy blends of high linoleic sunflower oil with selected cold pressed oils: Functionality, stability and antioxidative characteristics. Ind. Crop. Prod. 2013, 43, 65–72. [Google Scholar] [CrossRef]
- Rutckeviski, R.; Xavier-Junior, F.H.; Morais, A.R.; Alencar, E.N.; Amaral-Machado, L.; Genre, J.; Gondim, A.D.; Egito, E.S. Thermo-oxidative stability evaluation of bullfrog (rana catesbeiana shaw) oil. Molecules 2017, 22, 606. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.C.; Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Chong, W.T.; Boosroh, M.H. Production and comparative fuel properties of biodiesel from non-edible oils: Jatropha curcas, sterculia foetida and ceiba pentandra. Energy Convers. Manag. 2013, 73, 245–255. [Google Scholar] [CrossRef]
- Comission, C.A. Report of the Fourteenth Session of the Codex Committee on Fats and Oils; Codex Alimentarius Comission: London, UK, 1993; p. 195. [Google Scholar]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2012, 64, 175–193. [Google Scholar] [CrossRef]
- Shojaei, A.H. Buccal mucosa as a route for systemic drug delivery: A review. J. Pharm. Pharm. Sci. 1998, 1, 15–30. [Google Scholar] [PubMed]
- Rousseau, D. Fat crystals and emulsion stability—A review. Food Res. Int. 2000, 33, 3–14. [Google Scholar] [CrossRef]
- Mcclements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Binkhamis, K.; Haldane, D. An observation of altered morphology of candida albicans in vivo. Clin. Microbiol. Newsl. 2018, 40, 162–164. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, J.; Zhang, R.; Yuan, S.; Lu, Q.; Yu, Y. Colloid properties of hydrophobic modified alginate: Surface tension, ζ-potential, viscosity and emulsification. Carbohydr. Polym. 2018, 181, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Figueiro, F.; de Fraga Dias, A.; Teixeira, H.F.; Battastini, A.M.O.; Koester, L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.; Rocha, A.I.; Leal, P.; Estanqueiro, M.; Lobo, J.M.S. Development and characterization of mucoadhesive buccal gels containing lipid nanoparticles of ibuprofen. Int. J. Pharm. 2017, 533, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Marxen, E.; Mosgaard, M.D.; Pedersen, A.M.L.; Jacobsen, J. Mucin dispersions as a model for the oromucosal mucus layer in in vitro and ex vivo buccal permeability studies of small molecules. Eur. J. Pharm. Biopharm. 2017, 121, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Teubl, B.J.; Absenger, M.; Fröhlich, E.; Leitinger, G.; Zimmer, A.; Roblegg, E. The oral cavity as a biological barrier system: Design of an advanced buccal in vitro permeability model. Eur. J. Pharm. Biopharm. 2013, 84, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Abu-Huwaij, R.; Obaidat, R.M.; Sweidan, K.; Al-Hiari, Y. Formulation and in vitro evaluation of xanthan gum or carbopol 934-based mucoadhesive patches, loaded with nicotine. AAPS Pharmscitech. 2011, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, J.; Vinckier, I.; Ludwig, A. The use of xanthan gum in an ophthalmic liquid dosage form: Rheological characterization of the interaction with mucin. J. Pharm. Sci. 2002, 91, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Shiledar, R.R.; Tagalpallewar, A.A.; Kokare, C.R. Formulation and in vitro evaluation of xanthan gum-based bilayered mucoadhesive buccal patches of zolmitriptan. Carbohydr. Polym. 2014, 101, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Ahuja, M.; Mehta, H. Thiol derivatization of xanthan gum and its evaluation as a mucoadhesive polymer. Carbohydr. Polym. 2015, 131, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Klemetsrud, T.; Jonassen, H.; Hiorth, M.; Kjøniksen, A.L.; Smistad, G. Studies on pectin-coated liposomes and their interaction with mucin. Colloids Surf. B Biointerfaces 2013, 103, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McClements, D.J. Characterization of mucin–lipid droplet interactions: Influence on potential fate of fish oil-in-water emulsions under simulated gastrointestinal conditions. Food Hydrocoll. 2016, 56, 425–433. [Google Scholar] [CrossRef]
- Fonseca, F.N.; Betti, A.H.; Carvalho, F.C.; Gremião, M.P.D.; Dimer, F.A.; Guterres, S.S.; Tebaldi, M.L.; Rates, S.M.K.; Pohlmann, A.R. Mucoadhesive amphiphilic methacrylic copolymer-functionalized poly (ε-caprolactone) nanocapsules for nose-to-brain delivery of olanzapine. J. Biomed. Nanotechnol. 2015, 11, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, candida parapsilosis and candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, D.A.A.K.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaawi, Z.M.; Manfredi, M.; Waugh, A.C.W.; McCullough, M.J.; Jorge, J.; Scully, C.; Porter, S.R. Molecular characterization of Candida spp. Isolated from the oral cavities of patients from diverse clinical settings. Mol. Oral Microbiol. 2002, 17, 44–49. [Google Scholar] [CrossRef]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-candida activity of brazilian medicinal plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, B.P.G.L.; Dominici, V.A.; Urbano, I.A.; Silva, J.A.; Araújo, I.B.; Santos-Magalhães, N.S.; Silva, A.K.A.; Medeiros, A.C.; Oliveira, A.G.; Egito, E.S.T. Amphotericin b microemulsion reduces toxicity and maintains the efficacy as an antifungal product. J. Biomed. Nanotechnol. 2012, 8, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Dalmora, M.E.; Dalmora, S.L.; Oliveira, A.G. Inclusion complex of piroxicam with β-cyclodextrin and incorporation in cationic microemulsion. In vitro drug release and in vivo topical anti-inflammatory effect. Int. J. Pharm. 2001, 222, 45–55. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Streisand, J.B. Oral mucosal drug delivery. Clin. Pharmacokinet. 2002, 41, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Caon, T.; Pan, Y.; Simões, C.M.O.; Nicolazzo, J.A. Exploiting the buccal mucosa as an alternative route for the delivery of donepezil hydrochloride. J. Pharm. Sci. 2014, 103, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.F.; Liu, F.; Brown, M.B. Advances in oral transmucosal drug delivery. J. Control. Release 2011, 153, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, F.J.; Costa, R.J.O.; Cesário, F.R.A.S.; Rodrigues, L.B.; Costa, J.G.M.; Coutinho, H.D.M.; Galvao, H.B.F.; Menezes, I.R.A. Activity of essential oils of piper aduncum anf and cinnamomum zeylanicum by evaluating osmotic and morphologic fragility of erythrocytes. Eur. J. Integr. Med. 2016, 8, 505–512. [Google Scholar] [CrossRef]
- Sobrinho, A.C.N.; de Souza, E.B.; Rocha, M.F.G.; Albuquerque, M.R.J.R.; Bandeira, P.N.; Santos, H.S.; Paula-Cavalcante, C.S.; Oliveira, S.S.; Aragão, P.R.; Morais, S.M. Chemical composition, antioxidant, antifungal and hemolytic activities of essential oil from Baccharis trinervis (Lam.) Pers.(Asteraceae). Ind. Crop. Prod. 2016, 84, 108–115. [Google Scholar] [CrossRef]
- Li, W.; Lin, X.; Yang, Z.; Zhang, W.; Ren, T.; Qu, F.; Wang, Y.; Zhang, N.; Tang, X. A bufadienolide-loaded submicron emulsion for oral administration: Stability, antitumor efficacy and toxicity. Int. J. Pharm. 2015, 479, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Jin, J.; Pan, C.; Meng, L.; Tang, X. Bioavailability and pharmacokinetics of bufadienolides-loaded lipid microspheres after different administrations to rats. Eur. J. Lipid Sci. Technol. 2011, 113, 1095–1105. [Google Scholar] [CrossRef]


| Excipients | % (w/w) | Function | |
|---|---|---|---|
| Aqueous phase | Butylhydroxyanisole | 0.01 | Antioxidant |
| Sucralose | 0.10 | Sweetener | |
| Tutti-frutti flavoring | 0.10 | Flavoring | |
| Sodium benzoate | 0.20 | Antimicrobial preservative | |
| Xanthan gum | 0.30 | Stabilizing agent | |
| Acesulfame k | 0.40 | Sweetener | |
| Tween® 20 | 3.80 | Surfactant | |
| Propylene glycol | 5.00 | Humectant | |
| Distilled water | 73.87 | Disperser agent | |
| Oily phase | Butylhydroxytoluene (BHT) | 0.01 | Antioxidant |
| Propylparaben | 0.02 | Antimicrobial preservative | |
| Span® 80 | 2.20 | Surfactant | |
| Bullfrog Oil/Miglyol® 812 | 14.00 | Oil |
| Sample | Peak Force | Debonding Distance | Wma |
|---|---|---|---|
| mN ± SD | mm ± SD | mN·mm ± SD | |
| BBE | 10.15 ± 2.00 | 1752.36 ± 215.53 | 1494.04 ± 203.45 |
| BE | 7.44 ± 1.53 | 949.94 ± 92.72 | 1080.96 ± 204.68 |
| Mucin Concentration (µg·mL−1) | Mean Diameter (LD) ± SD (µm) | Zeta Potential ± SD (mV) | ||||
|---|---|---|---|---|---|---|
| Mucin Dispersion * | BBE ** | BE ** | Mucin Dispersion * | BBE ** | BE ** | |
| 0 | - | 0.320 ± 0.35 | 0.186 ± 0.02 | - | −38.53 ± 6.23 | −18.20 ± 1.42 |
| 200 | 0.349 ± 0.291 | 0.834 ± 0.02 | 1.138 ± 0.07 | −1.79 ± 0.02 | −18.10 ± 0.99 | −22.75 ± 0.49 |
| 250 | 0.432 ± 0.249 | 0.944 ± 0.05 | 1.056 ± 0.23 | −2.69 ± 0.05 | −19.30 ± 1.41 | −15.70 ± 2.26 |
| 350 | 0.779 ± 0.139 | 1.053 ± 0.02 | 1.228 ± 0.31 | −4.65 ± 0.52 | −23.00 ± 4.38 | −16.15 ± 2.19 |
| Yeast | MIC (mg·mL−1) | |
|---|---|---|
| Bullfrog Oil | Buccal Bullfrog Oil Emulsion | |
| Candida albicans ATCC 90029 | 0.50 | 1.00 |
| Candida dubliniensis CBS 7987 | - | 0.50 |
| Candida glabrata ATCC 2001 | - | 1.00 |
| Candida parapsilosis ATCC 22019 | 0.25 | 1.00 |
| Candida metapsilosis ATCC 96143 | 0.50 | 0.50 |
| Candida orthopsilosis ATCC 96139 | 0.50 | 1.00 |
| Candida tropicalis ATCC 13803 | 0.50 | 1.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira-Oliveira, S.S.; Amaral-Machado, L.; De Oliveira, W.N.; Alencar, É.N.; Zatta, K.C.; De Souza, L.B.F.C.; Medeiros, A.d.C.; Chaves, G.M.; Egito, E.S.T. Buccal Bullfrog (Rana catesbeiana Shaw) Oil Emulsion: A Mucoadhesive System Intended for Treatment of Oral Candidiasis. Pharmaceutics 2018, 10, 257. https://doi.org/10.3390/pharmaceutics10040257
Moreira-Oliveira SS, Amaral-Machado L, De Oliveira WN, Alencar ÉN, Zatta KC, De Souza LBFC, Medeiros AdC, Chaves GM, Egito EST. Buccal Bullfrog (Rana catesbeiana Shaw) Oil Emulsion: A Mucoadhesive System Intended for Treatment of Oral Candidiasis. Pharmaceutics. 2018; 10(4):257. https://doi.org/10.3390/pharmaceutics10040257
Chicago/Turabian StyleMoreira-Oliveira, Susiane S., Lucas Amaral-Machado, Wógenes Nunes De Oliveira, Éverton N. Alencar, Kelly Cristine Zatta, Luanda B. F. C. De Souza, Aldo da Cunha Medeiros, Guilherme Maranhão Chaves, and Eryvaldo S. T. Egito. 2018. "Buccal Bullfrog (Rana catesbeiana Shaw) Oil Emulsion: A Mucoadhesive System Intended for Treatment of Oral Candidiasis" Pharmaceutics 10, no. 4: 257. https://doi.org/10.3390/pharmaceutics10040257
APA StyleMoreira-Oliveira, S. S., Amaral-Machado, L., De Oliveira, W. N., Alencar, É. N., Zatta, K. C., De Souza, L. B. F. C., Medeiros, A. d. C., Chaves, G. M., & Egito, E. S. T. (2018). Buccal Bullfrog (Rana catesbeiana Shaw) Oil Emulsion: A Mucoadhesive System Intended for Treatment of Oral Candidiasis. Pharmaceutics, 10(4), 257. https://doi.org/10.3390/pharmaceutics10040257