Polyurethanes as New Excipients in Nail Therapeutics
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Nail Lacquers
2.2.2. Preliminary In Vitro Release of Terbinafine from Nail Lacquers Containing Different Concentrations of PU
2.2.3. In Vitro Characterization Studies: SEM, Adhesion Tests and Determination of Antifungal Activity
2.2.4. Drying Time for Nail Lacquer Therapeutics
2.2.5. Final Formulations—In Vitro Drug Permeation and Porosity Nail Studies
Permeation Studies
Porosity Measurement
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Tests for Select the Formulation with Better Condition to Release the Drugs
3.2. SEM Analysis
3.3. Adhesion Test
3.4. Nail Lacquer’s Drying Time
3.5. Antifungal Activity
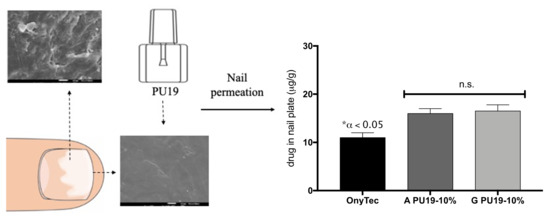
3.6. Nail Studies
Permeation Test
3.7. Porosity Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Souza, L.K.H.; Fernandes, O.F.L.; Passos, X.S.; Costa, C.R.; Lemos, J.A.; Silva, M.R.R. Epidemiological and mycological data of onychomycosis in Goiania, Brazil. Mycoses 2010, 53, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.W.; Ghannoum, M.A.; Elewski, B.E. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J. Am. Acad. Dermatol. 2004, 50, 748–752. [Google Scholar] [CrossRef]
- Garcia-Martos, P.; Dominguez, I.; Marin, P.; Linares, M.; Mira, J.; Calap, J. Onychomycoses caused by non-dermatophytic filamentous fungi in Cadiz. Enferm. Infecc. Microbiol. Clin. 2000, 18, 319–324. [Google Scholar] [PubMed]
- McAuley, W.J.; Jones, S.A.; Traynor, M.J.; Guesn, S.; Murdan, S.; Brown, M.B. An investigation of how fungal infection influences drug penetration through onychomycosis patient’s nail plates. Eur. J. Pharm. Biopharm. 2016, 102, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsayed, M.M.A.A. Development of topical therapeutics for management of onychomycosis and other nail disorders: A pharmaceutical perspective. J. Control. Release 2014, 199, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Barot, B.S.; Parejiya, P.B.; Patel, H.K.; Mehta, D.M.; Shelat, P.K. Drug delivery to the nail: Therapeutic options and challenges for onychomycosis. Crit. Rev. Ther. Drug Carrier Syst. 2014, 31, 459–494. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Herranz, U.; Bo, L.D.; Subissi, A. Nail penetration and predicted mycological efficacy of an innovative hydrosoluble ciclopirox nail lacquer vs. a standard amorolfine lacquer in healthy subjects. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e153–e158. [Google Scholar] [CrossRef]
- Monti, D.; Saccomani, L.; Chetoni, P.; Burgalassi, S.; Senesi, S.; Ghelardi, E.; Mailland, F. Hydrosoluble medicated nail lacquers: In vitro drug permeation and corresponding antimycotic activity. Br. J. Dermatol. 2010, 162, 311–317. [Google Scholar] [CrossRef]
- Nogueiras-Nieto, L.; Delgado-Charro, M.B.; Otero-Espinar, F.J. Thermogelling hydrogels of cyclodextrin/poloxamer polypseudorotaxanes as aqueous-based nail lacquers: Application to the delivery of triamcinolone acetonide and ciclopirox olamine. Eur. J. Pharm. Biopharm. 2013, 83, 370–377. [Google Scholar] [CrossRef]
- Kerai, L.V.; Hilton, S.; Murdan, S. UV-curable gel formulations: Potential drug carriers for the topical treatment of nail diseases. Int. J. Pharm. 2015, 492, 177–190. [Google Scholar] [CrossRef]
- Thatai, P.; Tiwary, A.K.; Sapra, B. Progressive Development in Experimental Models of Transungual Drug Delivery of Antifungal Agents. Int. J. Cosmet. Sci. 2015, 38, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuan, H.-C.; Chuang, W.-P.; Ma, C.-C.M.; Chiang, C.-L.; Wu, H.-L. Synthesis and characterization of a clay/waterborne polyurethane nanocomposite. J. Mater. Sci. 2005, 40, 179–185. [Google Scholar] [CrossRef]
- Täuber, A.; Müller-Goymann, C.C. In vitro permeation and penetration of ciclopirox olamine from poloxamer 407-based formulations–comparison of isolated human stratum corneum, bovine hoof plates and keratin films. Int. J. Pharm. 2015, 489, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Bohn, M.; Kraemer, K.T. Dermatopharmacology of ciclopirox nail lacquer topical solution 8% in the treatment of onychomycosis. J. Am. Acad. Dermatol. 2000, 43, S57–S69. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flör, C.; Mayr, A.; Perkhofer, S.; Hinterberger, G.; Hausdorfer, J.; Speth, C.; Fille, M. Activities of antifungal agents against yeasts and filamentous fungi: Assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 2008, 52, 3637–3641. [Google Scholar] [CrossRef] [PubMed]
- Hartmane, I.; Dervenice, A.; Mailland, F.; Mikazans, I.; Frisenda, L. Evaluation of safety profile, pharmacokinetics, and clinical benefit of an innovative terbinafine transungual solution (P-3058): A phase i study in patients with mild-to-moderate distal subungual onychomycosis. J. Am. Acad. Dermatol. 2013, 68, AB105. [Google Scholar] [CrossRef]
- Tanrıverdi, S.T.; Özer, Ö. Novel topical formulations of Terbinafine-HCl for treatment of onychomycosis. Eur. J. Pharm. Sci. 2013, 48, 628–636. [Google Scholar] [CrossRef]
- Siu, W.J.J.; Tatsumi, Y.; Senda, H.; Pillai, R.; Nakamura, T.; Sone, D.; Fothergill, A. Comparison of in vitro antifungal activities of efinaconazole and currently available antifungal agents against a variety of pathogenic fungi associated with onychomycosis. Antimicrob. Agents Chemother. 2013, 57, 1610–1616. [Google Scholar] [CrossRef]
- Valdes, B.S.G.; Serro, A.P.; Gordo, P.M.; Silva, A.; Gonçalves, L.; Salgado, A.; Marto, J.; Baltazar, D.; Santos, R.G.d.; Bordado, J.M.; et al. New Polyurethane Nail Lacquers for the Delivery of Terbinafine: Formulation and Antifungal Activity Evaluation. J. Pharm. Sci. 2017, 106, 1570–1577. [Google Scholar] [CrossRef]
- Salgado, A.; Raposo, S.; Marto, J.; Silva, A.N.; Simões, S.; Ribeiro, H.M. Mometasone furoate hydrogel for scalp use: In vitro and in vivo evaluation. Pharm. Dev. Technol. 2014, 19, 618–622. [Google Scholar] [CrossRef]
- ISO. EN ISO 2409, Paints and Varnishes, Cross-Cut Test; ISO: Geneva, Switzerland, 2013; Volume 3, pp. 4–13. [Google Scholar] [CrossRef]
- Arunprasad, K.; Narayanan, N.; Rajalakshmi, G. Preparation and Evaluation of Solid Dispersion of Terbinafine Hydrochloride. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 130–134. [Google Scholar]
- Nogueiras-Nieto, L.; Gómez-Amoza, J.L.; Delgado-Charro, M.B.; Otero-Espinar, F.J. Hydration and N-acetyl-l-cysteine alter the microstructure of human nail and bovine hoof: Implications for drug delivery. J. Control. Release 2011, 156, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Murdan, S.; Kerai, L.; Hossin, B. To what extent do in vitro tests correctly predict the in vivo residence of nail lacquers on the nail plate? J. Drug Deliv. Sci. Technol. 2015, 25, 23–28. [Google Scholar] [CrossRef]
- Baran, R.; Tosti, A.; Hartmane, I.; Altmeyer, P.; Hercogova, J.; Koudelkova, V.; Ruzicka, T.; Combemale, P.; Mikazans, I. An innovative water-soluble biopolymer improves efficacy of ciclopirox nail lacquer in the management of onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, H.N.; Vaka, S.R.K.; Madhav, N.V.S.; Chandra, H.; Murthy, S.N. Bilayered nail lacquer of terbinafine hydrochloride for treatment of onychomycosis. J. Pharm. Sci. 2010, 99, 4267–4276. [Google Scholar] [CrossRef] [PubMed]
- Kepka, S.W.; Lu, M.; Mo, Y.J.; Pfister, W.R.; Wang, H.-Y. Antifungal Nail Coat and Method of Use. Patent EP 1635770B1, 27 May 2009. [Google Scholar]
- Babapour, R. Treatment of Onychomycosis and Related Compositions. U.S. Patent 201002616A1, 4 October 2009. [Google Scholar]
- Gyurik, R. Pharmaceutical Composition. U.S. Patent 20030049307A1, 13 March 2003. [Google Scholar]
- Ryder, N.S.; Wagner, S.; Leitner, I. In vitro activities of terbinafine against cutaneous isolates of Candida albicans and other pathogenic yeasts. Antimicrob. Agents Chemother. 1998, 42, 1057–1061. [Google Scholar] [CrossRef]
- Petranyi, G.; Meingassner, J.G.; Mieth, H. Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob. Agents Chemother. 1987, 31, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, P.; Saini, T.R. Hydration of nail plate: A novel screening model for transungual drug permeation enhancers. Int. J. Pharm. 2012, 436, 179–182. [Google Scholar] [CrossRef]
- Khengar, R.H.; Jones, S.A.; Turner, R.B.; Forbes, B.; Brown, M.B. Nail swelling as a pre-formulation screen for the selection and optimisation of ungual penetration enhancers. Pharm. Res. 2007, 24, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, K.; Sugimoto, N.; Hosaka, S.; Katafuchi-Nagashima, M.; Arakawa, Y.; Tatsumi, Y.; Siu, W.J.; Pillai, R. The low keratin affinity of efinaconazole contributes to its nail penetration and fungicidal activity in topical onychomycosis treatment. Antimicrob. Agents Chemother. 2014, 58, 3837–3842. [Google Scholar] [CrossRef] [PubMed]
- Saeio, K.; Pongpaibul, Y.; Viernstein, H.; Okonogi, S. Factors influencing drug dissolution characteristics from hydrophilic polymer matrix tablet. Sci. Pharm. 2007, 75, 147–163. [Google Scholar] [CrossRef]





| Quantitative Composition (%, w/w) | |||||
|---|---|---|---|---|---|
| Formulation A PU19-10% TH | Formulation D PU19-15% TH | Formulation E PU19-20% TH | Formulation F PU19-25% TH | Formulation G PU19-10% CPX | |
| PU 19 | 10 | 15 | 20 | 25 | 10 |
| Terbinafine HCl (TH) | 1.0 | 1.0 | 1.0 | 1.0 | - |
| Ciclopirox (CPX) | - | - | - | - | 1.0 |
| Ethyl acetate | 7.8 | 7.2 | 6.8 | 6.3 | 17.8 |
| Butyl acetate | 10 | 9.6 | 9.0 | 8.5 | - |
| Ethanol | 71.2 | 67.2 | 63.2 | 59.2 | 71.2 |
| Formulation A PU19-10% | Formulation D PU19-15% | Formulation E PU19-20% | Formulation F PU19-25% | |
|---|---|---|---|---|
| Kosmeyer and Peppas | ||||
| k | 35.41 | 3.486 | 2.366 | 1.152 |
| n | 0.34 | 0.8924 | 0.6054 | 0.8864 |
| r2 | 0.9979 | 0.9615 | 0.9588 | 0.9662 |
| Higuchi | ||||
| k | 26.12 | 6.32 | 2.872 | 2.304 |
| r2 | 0.973 | 0.876 | 0.951 | 0.898 |
| Formulations | Viscosity (mPa.s) |
|---|---|
| Formulation A PU19-10% TH | 2.62 ± 0.04 |
| Formulation D PU19-15% TH | 4.70 ± 0.06 |
| Formulation E PU19-20% TH | 8.80 ± 0.11 |
| Formulation F PU19-25% TH | 17.03 ± 0.07 |
| Formulations | Time (min) |
|---|---|
| Formulation A PU19-10% TH | 9 |
| Formulation D PU19-15% TH | 10 |
| Formulation E PU19-20% TH | 15 |
| Formulation F PU19-25% TH | 16 |
| Formulation G PU19-10% CPX | 7 |
| Ony-Tec® | 13 |
| Formulations | Inhibition Zone (mm) | |
|---|---|---|
| Candida albicans ATCC 10240 | Aspergillus brasiliensis ATCC 16404 | |
| Formulation A PU19 10% TH | 38.4 ± 3.6 | 25.0 ± 0.4 |
| Formulation D PU19 15% TH | 29.7 ± 0.4 | 23.7 ± 0.6 |
| Formulation E PU19 20% TH | 29.0 ± 0.6 | 23.2 ± 0.1 |
| Formulation F PU19 25% TH | 26.2 ± 1.0 | 21.1 ± 1.7 |
| Formulation G PU19 10% CPX | 32.3 ± 1.3 | 26.7 ± 0.3 |
| Solution TH (1%) | 21.4 ± 1.8 | 32.0 ± 0.8 |
| Solution CPX (1%) | 29.0 ± 1.1 | 31.4 ± 0.4 |
| Co (mg/mL) | Acum72h mg/cm2 | Acum264h µg/cm2 | % Dose Delivered | Japp 48–264 h | |
| Ony-Tec® | 80 | 0.19 ± 0.17 | 0.29 ± 0.16 | 0.01 ± 0.005 | 14.1 ± 2.68 |
| Formulation G PU19-10% CPX | 10 | 0.51 ± 0.35 | 0.87 ± 0.50 | 0.21 ± 0.12 | 50.5 ± 4.12 |
| Formulation A PU19-10% TH | 10 | 0.48 ± 0.03 | 0.80 ± 0.11 | 0.20 ± 0.03 | 36.5 ± 3.69 |
| Porosity (%) | Correlation | Connectivity | Water Permeability (mD) | |
|---|---|---|---|---|
| Untreated nails | 6.83 | 0.795 | 5.02 | 1.5775 × 10−6 |
| Ony-Tec® | 6.75 | 0.796 | 5.02 | 1.5417 × 10−6 |
| Formulation A PU19 10% TH | 6.75 | 0.795 | 5.02 | 1.5775 × 10−6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdes, B.S.G.; Serro, A.P.; Marto, J.; Galhano dos Santos, R.; Cutrín Gómez, E.; Otero-Espinar, F.J.; Moura Bordado, J.; Margarida Ribeiro, H. Polyurethanes as New Excipients in Nail Therapeutics. Pharmaceutics 2018, 10, 276. https://doi.org/10.3390/pharmaceutics10040276
Valdes BSG, Serro AP, Marto J, Galhano dos Santos R, Cutrín Gómez E, Otero-Espinar FJ, Moura Bordado J, Margarida Ribeiro H. Polyurethanes as New Excipients in Nail Therapeutics. Pharmaceutics. 2018; 10(4):276. https://doi.org/10.3390/pharmaceutics10040276
Chicago/Turabian StyleValdes, Barbara S. Gregorí, Ana Paula Serro, Joana Marto, Rui Galhano dos Santos, Elena Cutrín Gómez, Francisco J. Otero-Espinar, João Moura Bordado, and Helena Margarida Ribeiro. 2018. "Polyurethanes as New Excipients in Nail Therapeutics" Pharmaceutics 10, no. 4: 276. https://doi.org/10.3390/pharmaceutics10040276
APA StyleValdes, B. S. G., Serro, A. P., Marto, J., Galhano dos Santos, R., Cutrín Gómez, E., Otero-Espinar, F. J., Moura Bordado, J., & Margarida Ribeiro, H. (2018). Polyurethanes as New Excipients in Nail Therapeutics. Pharmaceutics, 10(4), 276. https://doi.org/10.3390/pharmaceutics10040276