Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility
Abstract
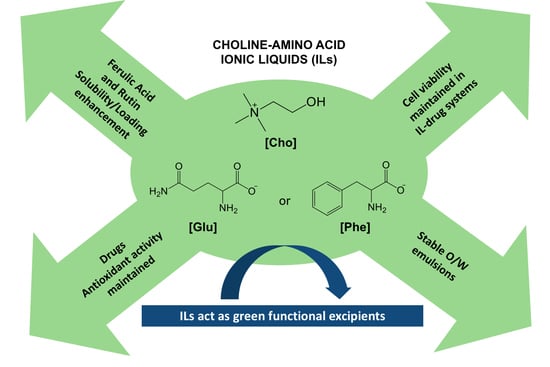
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ionic Liquids Synthesis
2.3. Solubility Studies
2.4. Cell Culture
2.5. Crystal Violet (CV) Staining Assay
2.6. Radical Scavenging Assay with DPPH Radical (DPPH Assay)
2.7. Preparation of O/W Emulsions
2.8. Accelerated Stability Studies of the O/W Emulsions
2.9. Real-Time Stability Studies of the O/W Emulsions
3. Results and Discussion
3.1. Solubility Studies
3.2. Impact of ILs Combined with Ferulic Acid and Rutin on Cell Viability
3.3. Antioxidant Activity by DPPH Assay
3.4. Drug Incorporation in O/W Emulsions and Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Di, L.; Fish, P.V.; Mano, T. Bridging solubility between drug discovery and development. Drug Discov. Today 2012, 17, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xing, H.; Zhao, Y.; Ma, Z. Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs. Pharmaceutics 2018, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, Y.; Zhao, B.; Liang, G.; Liu, S.; Liu, X.-L.; Yu, D.-G. Fast Dissolving of Ferulic Acid via Electrospun Ternary Amorphous Composites Produced by a Coaxial Process. Pharmaceutics 2018, 10, 115. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Juère, E.; Florek, J.; Bouchoucha, M.; Jambhrunkar, S.; Wong, K.Y.; Popat, A.; Kleitz, F. In Vitro Dissolution, Cellular Membrane Permeability, and Anti-Inflammatory Response of Resveratrol-Encapsulated Mesoporous Silica Nanoparticles. Mol. Pharm. 2017, 14, 4431–4441. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.C.; Gavin, P.; Libinaki, R.; Ramirez, G.; Boyd, B.J. A new lipid excipient, phosphorylated tocopherol mixture, TPM enhances the solubilisation and oral bioavailability of poorly water soluble CoQ10in a lipid formulation. J. Control. Release 2017, 268, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Singh Malik, D.; Mital, N.; Kaur, G. Topical drug delivery systems: A patent review. Expert Opin. Ther. Pat. 2016, 26, 213–228. [Google Scholar] [CrossRef]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and transdermal drug delivery systems: Current and future prospects. Drug Deliv. 2006, 13, 175–187. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B.S.; Saha, K. Advances in Topical Drug Delivery System: Micro to Nanofibrous Structures. J. Nanosci. Nanotechnol. 2014, 14, 853–867. [Google Scholar] [CrossRef]
- Dobler, D.; Schmidts, T.; Klingenhoefer, I.; Runkel, F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. 2013, 441, 620–627. [Google Scholar] [CrossRef]
- Gouveia, W.; Jorge, T.F.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; Almeida, T.V.; Araújo, M.E.M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Balk, A.; Holzgrabe, U.; Meinel, L. ‘Pro et contra’ ionic liquid drugs—Challenges and opportunities for pharmaceutical translation. Eur. J. Pharm. Biopharm. 2015, 94, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.; Branco, L.C.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Ionic liquids as active pharmaceutical ingredients. ChemMedChem 2011, 6, 975–985. [Google Scholar] [CrossRef]
- Santos de Almeida, T.; Júlio, A.; Saraiva, N.; Fernandes, A.S.; Araújo, M.E.M.; Baby, A.R.; Rosado, C.; Mota, J.P. Choline- versus imidazole-based ionic liquids as functional ingredients in topical delivery systems: Cytotoxicity, solubility, and skin permeation studies. Drug Dev. Ind. Pharm. 2017, 43, 1858–1865. [Google Scholar] [CrossRef]
- Agatemor, C.; Ibsen, K.N.; Tanner, E.E.L.; Mitragotri, S. Ionic liquids for addressing unmet needs in healthcare. Bioeng. Transl. Med. 2018, 7–25. [Google Scholar] [CrossRef]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Mizuuchi, H.; Jaitely, V.; Murdan, S.; Florence, A.T. Room temperature ionic liquids and their mixtures: Potential pharmaceutical solvents. Eur. J. Pharm. Sci. 2008, 33, 326–331. [Google Scholar] [CrossRef]
- Quraish, K.S.; Bustam, M.A.; Krishnan, S.; Aminuddin, N.F.; Azeezah, N.; Ghani, N.A.; Uemura, Y.; Lévêque, J.M. Ionic liquids toxicity on fresh water microalgae, Scenedesmus quadricauda, Chlorella vulgaris & Botryococcus braunii; selection criterion for use in a two-phase partitioning bioreactor (TPPBR). Chemosphere 2017, 184, 642–651. [Google Scholar] [CrossRef]
- Fatemi, M.H.; Izadiyan, P. Cytotoxicity estimation of ionic liquids based on their effective structural features. Chemosphere 2011, 84, 553–563. [Google Scholar] [CrossRef]
- Montalbán, M.G.; Hidalgo, J.M.; Collado-González, M.; Díaz Baños, F.G.; Víllora, G. Assessing chemical toxicity of ionic liquids on Vibrio fischeri: Correlation with structure and composition. Chemosphere 2016, 155, 405–414. [Google Scholar] [CrossRef]
- Ossowicz, P.; Janus, E. Studies on thermal stability of amino acid ionic liquids. Chemik 2016, 70, 483–484. [Google Scholar]
- Moniruzzaman, M.; Kamiya, N.; Goto, M. Ionic liquid based microemulsion with pharmaceutically accepted components: Formulation and potential applications. J. Colloid Interface Sci. 2010, 352, 136–142. [Google Scholar] [CrossRef]
- Heckenbach, M.E.; Romero, F.N.; Green, M.D.; Halden, R.U. Meta-analysis of ionic liquid literature and toxicology. Chemosphere 2016, 150, 266–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.I.N.; Lee, S.; Kim, K.; Joo, D.A.H.Y.E.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Kwon, S.B.I.N.; Kim, M.I.N.J.; et al. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 357–363. [Google Scholar] [CrossRef]
- Costa, J.G.; Saraiva, N.; Guerreiro, P.S.; Louro, H.; Silva, M.J.; Miranda, J.P.; Castro, M.; Batinic-Haberle, I.; Fernandes, A.S.; Oliveira, N.G. Ochratoxin A-induced cytotoxicity, genotoxicity and reactive oxygen species in kidney cells: An integrative approach of complementary endpoints. Food Chem. Toxicol. 2016, 87, 65–76. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Serejo, J.; Gaspar, J.; Cabral, F.; Bettencourt, A.F.; Rueff, J.; Castro, M.; Costa, J.; Oliveira, N.G. Oxidative injury in V79 Chinese hamster cells: Protective role of the superoxide dismutase mimetic MnTM-4-PyP. Cell Biol. Toxicol. 2010, 26, 91–101. [Google Scholar] [CrossRef]
- Guerreiro, P.S.; Corvacho, E.; Costa, J.G.; Saraiva, N.; Fernandes, A.S.; Castro, M.; Miranda, J.P.; Oliveira, N.G. The APE1 redox inhibitor E3330 reduces collective cell migration of human breast cancer cells and decreases chemoinvasion and colony formation when combined with docetaxel. Chem. Biol. Drug Des. 2017, 90, 561–571. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- De Oliveira, C.A.; Peres, D.D.A.; Graziola, F.; Chacra, N.A.B.; De Araújo, G.L.B.; Flórido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.R.; Rodrigues, L.M.; et al. Cutaneous biocompatible rutin-loaded gelatin-based nanoparticles increase the SPF of the association of UVA and UVB filters. Eur. J. Pharm. Sci. 2016, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, J.L.; Pearce, S.E.M. Handbook of Cosmetic Science and Technology, 1st ed.; Elsevier Advanced Technology, Ed.; Elsevier Science: Oxford, UK, 1993. [Google Scholar]
- Pereira, M.; Pereira, N.; Rosado, C.; de Oliveira, C.A.; Peres, D.A.; Araújo, M.E.; Velasco, M.V.R.; Baby, A.R.; Mota, J.; Almeida, T.S. Photostabilization of sunscreens by incorporation of tea as the external phase Fotoestabilização de protectores solares por incorporação de chás como fase externa. Biomed. Biopharm. Res. 2015, 12, 107–116. [Google Scholar]
- Mota, L.; Queimada, J.; Pinho, P. Aqueous Solubility of Some Natural Phenolic Compounds. Ind. Eng. Chem. Res. 2008, 47, 5182–5189. [Google Scholar] [CrossRef] [Green Version]
- Nagarjuna, S.; Murthy, T.E.G.K.; Rao, A.S. Solubility Data of Naringin and Rutin in Different Ph Media Using Uv Visible Spectrophotometer. J. Pharm. Sci. Innov. 2016, 5, 63–65. [Google Scholar] [CrossRef]
- Yang, G.W.; Jiang, J.S.; Lu, W.Q. Ferulic acid exerts anti-angiogenic and anti-tumor activity by targeting fibroblast growth factor receptor 1-mediated angiogenesis. Int. J. Mol. Sci. 2015, 16, 24011–24031. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wu, Q.; Yang, S.H. Ferulic acid promoting apoptosis in human osteosarcoma cell lines. Pak. J. Med. Sci. 2017, 33, 127–131. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, S.; Li, N.; Ho, A.S.W.; Kiang, K.M.Y.; Zhang, X.; Cheng, Y.S.; Poon, M.W.; Lee, D.; Pu, J.K.S.; et al. Rutin increases the cytotoxicity of temozolomide in glioblastoma via autophagy inhibition. J. Neurooncol. 2017, 132, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Maistro, E.L.; Angeli, J.P.; Andrade, S.F.; Mantovani, M.S. In vitro genotoxicity assessment of caffeic, cinnamic and ferulic acids. Genet. Mol. Res. 2011, 10, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Cristina Marcarini, J.; Ferreira Tsuboy, M.S.; Cabral Luiz, R.; Regina Ribeiro, L.; Beatriz Hoffmann-Campo, C.; Ségio Mantovani, M. Investigation of cytotoxic, apoptosis-inducing, genotoxic and protective effects of the flavonoid rutin in HTC hepatic cells. Exp. Toxicol. Pathol. 2011, 63, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a Quercetin Glycoside, Restores Chemosensitivity in Human Breast Cancer Cells. Phytother. Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef]
- Serafim, T.; Garrido, J.; Milhazes, N.; Borges, F.; Tavares, E.; Holy, J.; Oliveira, P.J. Lipophilic caffeic and ferulic acid derivatives presenting cytotoxicity against human breast cancer cells. Chem. Res. Toxicol. 2011, 24, 763–774. [Google Scholar] [CrossRef]
- Chen, J.; Lu, L.; Feng, Y.; Wang, H.; Dai, L.; Li, Y.; Zhang, P. PKD2 mediates multi-drug resistance in breast cancer cells through modulation of P-glycoprotein expression. Cancer Lett. 2011, 300, 48–56. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Recent Advances in Indian Herbal Drug Research Guest Editor: Thomas Paul Asir Devasagayam Ferulic Acid: Therapeutic Potential Through Its Antioxidant Property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [Green Version]




| Drug | Composition | O/W Emulsions | ||
|---|---|---|---|---|
| Control | [Cho][Phe] | [Cho][Glu] | ||
| Ferulic Acid | Crodafos® CES | 4 or 6 | 4 | 6 |
| Isopropyl myristate | 2 | 2 | 2 | |
| Butylated hydroxytoluene (BHT) | 0.1 | 0.1 | 0.1 | |
| Ethylenediaminetetraacetic acid disodium dihydrate (EDTA Na2) | 0.1 | 0.1 | 0.1 | |
| Propylene glycol (PG) | 5 | 5 | 5 | |
| Polyethylene glycol (PEG 400) | 5 | 5 | 5 | |
| Parabens solution | 1 | 1 | 1 | |
| Ferulic acid | – | 0.13 | 0.12 | |
| IL | – | 0.2 | 0.2 | |
| Triethanolamine | q.s. to pH = 5 q.s. to 100 | |||
| Deionized water | ||||
| Rutin | Crodafos® CES | 4 or 6 | 4 | 6 |
| Isopropyl myristate | 2 | 2 | 2 | |
| Butylated hydroxytoluene (BHT) | 0.1 | 0.1 | 0.1 | |
| Ethylenediaminetetraacetic acid disodium dihydrate (EDTA Na2) | 0.1 | 0.1 | 0.1 | |
| Propylene glycol (PG) | 5 | 5 | 5 | |
| Polyethylene glycol (PEG 400) | 5 | 5 | 5 | |
| Parabens solution | 1 | 1 | 1 | |
| Rutin | – | 0.115 | 0.068 | |
| IL | – | 0.2 | 0.2 | |
| Triethanolamine | q.s. to pH = 5 q.s. to 100 | |||
| Deionized water | ||||
| Solvent | Drug Concentration (mg/mL) | RSA (%) | |
|---|---|---|---|
| Ferulic Acid | Water | 0.64 | 78.5 ± 2.6 a |
| Water:[Cho][Phe] | 0.64 | 78.1 ± 4.3 a | |
| 1.30 | 89.9 ± 1.8 b | ||
| Water:[Cho][Glu] | 0.64 | 79.9 ± 1.1 a | |
| 1.20 | 88.8 ± 0.5 b | ||
| Rutin | Water | 0.20 | 20.5 ± 1.3 a |
| Water:[Cho][Phe] | 0.20 | 20.4 ± 0.7 a | |
| 1.15 | 93.6 ± 0.3 b | ||
| Water:[Cho][Glu] | 0.20 | 19.5 ± 0.2 a | |
| 0.68 | 56.2 ± 0.4 c |
| Formulation | Crodafos® CES (%) | Accelerated Stability Studies | Stability Studies (after 3 Months) | RT Stability Studies (after 3 Months) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Zero | After 5 cycles | −10 °C | 45 °C | RT | |||||||
| pH | Visc. (mPas) | pH | Visc. (mPas) | pH | Visc. (mPas) | pH | Visc. (mPas) | pH | Visc. (mPas) | ||
| Control | 4 | 4.99 | 10,260 | 5.00 | 11,503 | 4.8 | 12,400 | 4.9 | 12,300 | 4.8 | 12,350 |
| FA/[Cho][Phe] | 5.00 | 12,500 | 4.68 | 14,700 | 4.5 | 15,530 | 4.5 | 15,600 | 4.5 | 15,400 | |
| Rut/[Cho][Phe] | 5.02 | 14,300 | 4.35 | 19,630 | 4.7 | 20,000 | 4.8 | 19,980 | 4.8 | 20,060 | |
| Control | 6 | 4.99 | 17,130 | 5.00 | 19,100 | 4.7 | 20,200 | 4.8 | 20,140 | 4.8 | 20,100 |
| FA/[Cho][Glu] | 5.05 | 19,600 | 4.50 | 21,450 | 4.45 | 22,600 | 4.6 | 22,660 | 4.5 | 22,500 | |
| Rut/[Cho][Glu] | 4.99 | 19,000 | 4.50 | 22,800 | 4.5 | 23,130 | 4.6 | 23,000 | 4.6 | 23,060 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caparica, R.; Júlio, A.; Baby, A.R.; Araújo, M.E.M.; Fernandes, A.S.; Costa, J.G.; Santos de Almeida, T. Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility. Pharmaceutics 2018, 10, 288. https://doi.org/10.3390/pharmaceutics10040288
Caparica R, Júlio A, Baby AR, Araújo MEM, Fernandes AS, Costa JG, Santos de Almeida T. Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility. Pharmaceutics. 2018; 10(4):288. https://doi.org/10.3390/pharmaceutics10040288
Chicago/Turabian StyleCaparica, Rita, Ana Júlio, André Rolim Baby, Maria Eduarda Machado Araújo, Ana Sofia Fernandes, João Guilherme Costa, and Tânia Santos de Almeida. 2018. "Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility" Pharmaceutics 10, no. 4: 288. https://doi.org/10.3390/pharmaceutics10040288
APA StyleCaparica, R., Júlio, A., Baby, A. R., Araújo, M. E. M., Fernandes, A. S., Costa, J. G., & Santos de Almeida, T. (2018). Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility. Pharmaceutics, 10(4), 288. https://doi.org/10.3390/pharmaceutics10040288