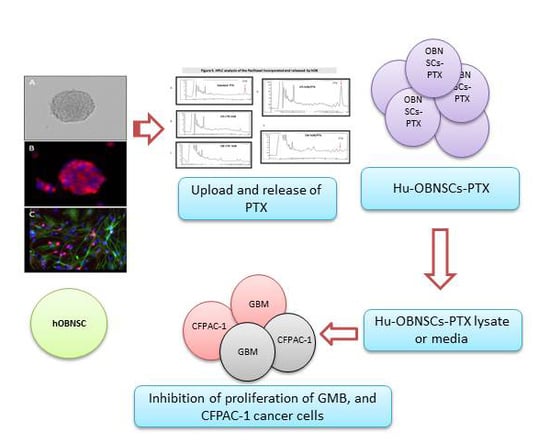
Human Olfactory Bulb Neural Stem Cells (Hu-OBNSCs) Can Be Loaded with Paclitaxel and Used to Inhibit Glioblastoma Cell Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Human Olfactory Bulb NSCs Isolation, Culturing and Immunocytochemical Analysis
2.3. Immunocytochemical Analysis
2.4. Harvest of G-CSC Conditioned Medium (CM G-CSC)
2.5. Cell Invasion Assay
2.6. Sensitivity of Hu-OBNSCs1 and Hu-OBNSCs2 to Paclitaxel
2.7. Tumor Cells and Wharton’s Jelly Mesenchymal Stem Cells
2.8. Paclitaxel Loading of Human Olfactory Bulb Cells
2.9. In Vitro Anticancer Assay
2.10. High Performance Liquid Chromatogrphy (HPLC) Analysis
2.11. Statistical Analysis
3. Results
3.1. Morphology of Hu-OBNSCs Neurospheres, Immunecytochemical Analysis in Proliferation and Differentiation Conditions
3.2. Invasion Ability of Hu-OBNSCs Versus WJ-MSCs
3.3. Sensitivity of Hu-OBNSCs to Paclitaxel
3.4. Efficiency of PTX Incorporation and Anticancer Activity of PTX Released by Primed h-OB Cells
3.5. In Vitro Efficacy Against Human Glioblastoma Cells
3.6. HPLC Analysis of PTX Incorporated and Released by Hu-OBNSCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mooney, R.; Hammad, M.; Batalla-Covello, J.; Abdul Majid, A.; Aboody, K.S. Concise Review: Neural Stem Cell-Mediated Targeted Cancer Therapies. Stem Cells Transl. Med. 2018, 7, 740–747. [Google Scholar] [CrossRef]
- Marei, H.E.; Farag, A.; Althani, A.; Afifi, N.; Abd-Elmaksoud, A.; Lashen, S.; Rezk, S.; Pallini, R.; Casalbore, P.; Cenciarelli, C. Human olfactory bulb neural stem cells expressing hNGF restore cognitive deficit in Alzheimer’s disease rat model. J. Cell. Physiol. 2015, 230, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.; Althani, A.; Rezk, S.; Farag, A.; Lashen, S.; Afifi, N.; Abd-Elmaksoud, A.; Pallini, R.; Casalbore, P.; Cenciarelli, C. Therapeutic potential of human olfactory bulb neural stem cells for spinal cord injury in rats. Spinal Cord 2016, 54, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Lashen, S.; Farag, A.; Althani, A.; Afifi, N.; Rezk, S.; Pallini, R.; Casalbore, P.; Cenciarelli, C. Human olfactory bulb neural stem cells mitigate movement disorders in a rat model of Parkinson’s disease. J. Cell. Physiol. 2015, 230, 1614–1629. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, M.; Colognato, R.; Rimoldi, M.; Rizzetto, M.; Sisto, F.; Cocce, V.; Bonomi, A.; Parati, E.; Alessandri, G.; Bagnati, R. Mesenchymal Stromal Cells Uptake and Release Paclitaxel without Reducing its Anticancer Activity. Anti-Cancer Agents Med. Chem. 2015, 15, 400–405. [Google Scholar] [CrossRef]
- Casalbore, P.; Budoni, M.; Ricci-Vitiani, L.; Cenciarelli, C.; Petrucci, G.; Milazzo, L.; Montano, N.; Tabolacci, E.; Maira, G.; Larocca, L.M. Tumorigenic potential of olfactory bulb-derived human adult neural stem cells associates with activation of TERT and NOTCH1. PLoS ONE 2009, 4, e4434. [Google Scholar] [CrossRef]
- Cenciarelli, C.; Marei, H.E.; Zonfrillo, M.; Pierimarchi, P.; Paldino, E.; Casalbore, P.; Felsani, A.; Vescovi, A.L.; Maira, G.; Mangiola, A. PDGF receptor alpha inhibition induces apoptosis in glioblastoma cancer stem cells refractory to anti-Notch and anti-EGFR treatment. Mol. Cancer 2014, 13, 247. [Google Scholar] [CrossRef] [Green Version]
- Ponten, J.; Macintyre, E.H. Long term culture of normal and neoplastic human glia. Acta Pathol. Microbiol. Scand. 1968, 74, 465–486. [Google Scholar] [CrossRef]
- Allen, M.; Bjerke, M.; Edlund, H.; Nelander, S.; Westermark, B. Origin of the U87MG glioma cell line: Good news and bad news. Sci. Transl. Med. 2016, 8, 354re353. [Google Scholar] [CrossRef]
- McIntosh, J.C.; Schoumacher, R.A.; Tiller, R.E. Pancreatic adenocarcinoma in a patient with cystic fibrosis. Am. J. Med. 1988, 85, 592. [Google Scholar] [CrossRef]
- Paldino, E.; Cenciarelli, C.; Giampaolo, A.; Milazzo, L.; Pescatori, M.; Hassan, H.J.; Casalbore, P. Induction of dopaminergic neurons from human Wharton’s jelly mesenchymal stem cell by forskolin. J. Cell. Physiol. 2014, 229, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Pessina, A.; Bonomi, A.; Coccè, V.; Invernici, G.; Navone, S.; Cavicchini, L.; Sisto, F.; Ferrari, M.; Viganò, L.; Locatelli, A. Mesenchymal stromal cells primed with paclitaxel provide a new approach for cancer therapy. PLoS ONE 2011, 6, e28321. [Google Scholar] [CrossRef]
- Bonomi, A.; Silini, A.; Vertua, E.; Signoroni, P.B.; Coccè, V.; Cavicchini, L.; Sisto, F.; Alessandri, G.; Pessina, A.; Parolini, O. Human amniotic mesenchymal stromal cells (hAMSCs) as potential vehicles for drug delivery in cancer therapy: An in vitro study. Stem Cell Res. Ther. 2015, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Brini, A.T.; Coccè, V.; Ferreira, L.M.J.; Giannasi, C.; Cossellu, G.; Giannì, A.B.; Angiero, F.; Bonomi, A.; Pascucci, L.; Falchetti, M.L. Cell-mediated drug delivery by gingival interdental papilla mesenchymal stromal cells (GinPa-MSCs) loaded with paclitaxel. Expert Opin. Drug Deliv. 2016, 13, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Coccè, V.; Farronato, D.; Brini, A.T.; Masia, C.; Giannì, A.B.; Piovani, G.; Sisto, F.; Alessandri, G.; Angiero, F.; Pessina, A. Drug Loaded Gingival Mesenchymal Stromal Cells (GinPa-MSCs) Inhibit In Vitro Proliferation of Oral Squamous Cell Carcinoma. Sci. Rep. 2017, 7, 9376. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple method of estimating fifty per cent endpoints 1 2. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Bonomi, A.; Coccè, V.; Cavicchini, L.; Sisto, F.; Dossena, M.; Balzarini, P.; Portolani, N.; Ciusani, E.; Parati, E.; Alessandri, G. Adipose tissue-derived stromal cells primed in vitro with paclitaxel acquire anti-tumor activity. Int. J. Immunopathol. Pharmacol. 2013, 26, 33–41. [Google Scholar] [CrossRef]
- Kumar, G.; Ray, S.; Walle, T.; Huang, Y.; Willingham, M.; Self, S.; Bhalla, K. Comparative in vitro cytotoxic effects of taxol and its major human metabolite 6α-hydroxytaxol. Cancer Chemother. Pharmacol. 1995, 36, 129–135. [Google Scholar] [CrossRef]
- Magatti, M.; Munari, S.; Vertua, E.; Parolini, O. Amniotic membrane-derived cells inhibit proliferation of cancer cell lines by inducing cell cycle arrest. J. Cell. Mol. Med. 2012, 16, 2208–2218. [Google Scholar] [CrossRef] [Green Version]
- Marei, H.E.; Althani, A.; Afifi, N.; Michetti, F.; Pescatori, M.; Pallini, R.; Casalbore, P.; Cenciarelli, C.; Schwartz, P.; Ahmed, A.-E. Gene expression profiling of embryonic human neural stem cells and dopaminergic neurons from adult human substantia nigra. PLoS ONE 2011, 6, e28420. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Ahmed, A.-E.; Michetti, F.; Pescatori, M.; Pallini, R.; Casalbore, P.; Cenciarelli, C.; Elhadidy, M. Gene expression profile of adult human olfactory bulb and embryonic neural stem cell suggests distinct signaling pathways and epigenetic control. PLoS ONE 2012, 7, e33542. [Google Scholar] [CrossRef]
- Marei, H.E.; Ahmed, A.-E. Transcription factors expressed in embryonic and adult olfactory bulb neural stem cells reveal distinct proliferation, differentiation and epigenetic control. Genomics 2013, 101, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marei, H.E.; Althani, A.; Afifi, N.; Abd-Elmaksoud, A.; Bernardini, C.; Michetti, F.; Barba, M.; Pescatori, M.; Maira, G.; Paldino, E. Over-expression of hNGF in adult human olfactory bulb neural stem cells promotes cell growth and oligodendrocytic differentiation. PLoS ONE 2013, 8, e82206. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Elnegiry, A.A.; Zaghloul, A.; Althani, A.; Afifi, N.; Abd-Elmaksoud, A.; Farag, A.; Lashen, S.; Rezk, S.; Shouman, Z. Nanotubes impregnated human olfactory bulb neural stem cells promote neuronal differentiation in trimethyltin-induced neurodegeneration rat model. J. Cell. Physiol. 2017, 232, 3586–3597. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.; Shouman, Z.; Althani, A.; Afifi, N.; Abd-Elmaksoud, A.; Lashen, S.; Hasan, A.; Caceci, T.; Rizzi, R.; Cenciarelli, C. Differentiation of human olfactory bulb-derived neural stem cell towards oligodendrocyte development. J. Cell. Physiol. 2018, 233, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Pessina, A.; Coccè, V.; Bonomi, A.; Cavicchini, L.; Sisto, F.; Ferrari, M.; Ciusani, E.; Navone, S.; Marfia, G.; Parati, E. Human skin-derived fibroblasts acquire in vitro anti-tumor potential after priming with Paclitaxel. Anti-Cancer Agents Med. Chem. 2013, 13, 523–530. [Google Scholar]
- de Weger, V.A.; Beijnen, J.H.; Schellens, J.H. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—A review. Anti-Cancer Drugs 2014, 25, 488–494. [Google Scholar] [CrossRef]
- Bocci, G.; Di Paolo, A.; Danesi, R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis 2013, 16, 481–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlahovic, G.; Karantza, V.; Wang, D.; Cosgrove, D.; Rudersdorf, N.; Yang, J.; Xiong, H.; Busman, T.; Mabry, M. A phase I safety and pharmacokinetic study of ABT-263 in combination with carboplatin/paclitaxel in the treatment of patients with solid tumors. Investig. New Drugs 2014, 32, 976–984. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Barry, W.T.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; Albain, K.S.; Rugo, H.S.; Ellis, M.; Shapira, I. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N. Engl. J. Med. 2015, 372, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Vähäkangas, K.; Myllynen, P. Drug transporters in the human blood-placental barrier. Br. J. Pharmacol. 2009, 158, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Shiverick, K.; Slikker, W.; Rogerson, S.; Miller, R. Drugs and the placenta—A workshop report. Placenta 2003, 24, S55–S59. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, L.; Gupta, A.; Vethanayagam, R.R.; Zhang, Y.; Unadkat, J.D.; Mao, Q. Regulation of BCRP/ABCG2 expression by progesterone and 17β-estradiol in human placental BeWo cells. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E798–E807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novotna, M.; Libra, A.; Kopecky, M.; Pavek, P.; Fendrich, Z.; Semecky, V.; Staud, F. P-glycoprotein expression and distribution in the rat placenta during pregnancy. Reprod. Toxicol. 2004, 18, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-Y.; Lee, H.-E.; Kang, Y.-S. Identification of p-glycoprotein and transport mechanism of Paclitaxel in syncytiotrophoblast cells. Biomol. Ther. 2014, 22, 68. [Google Scholar] [CrossRef]
- Hemauer, S.J.; Patrikeeva, S.L.; Nanovskaya, T.N.; Hankins, G.D.; Ahmed, M.S. Opiates inhibit paclitaxel uptake by P-glycoprotein in preparations of human placental inside-out vesicles. Biochem. Pharmacol. 2009, 78, 1272–1278. [Google Scholar] [CrossRef] [Green Version]
- Hemauer, S.J.; Nanovskaya, T.N.; Abdel-Rahman, S.Z.; Patrikeeva, S.L.; Hankins, G.D.; Ahmed, M.S. Modulation of human placental P-glycoprotein expression and activity by MDR1 gene polymorphisms. Biochem. Pharmacol. 2010, 79, 921–925. [Google Scholar] [CrossRef] [Green Version]
- Giannakakou, P.; Sackett, D.L.; Kang, Y.-K.; Zhan, Z.; Buters, J.T.; Fojo, T.; Poruchynsky, M.S. Paclitaxel-resistant human ovarian cancer cells have mutant β-tubulins that exhibit impaired paclitaxel-driven polymerization. J. Biol. Chem. 1997, 272, 17118–17125. [Google Scholar] [CrossRef]
- Kavallaris, M.; Kuo, D.Y.-S.; Burkhart, C.A.; Regl, D.L.; Norris, M.D.; Haber, M.; Horwitz, S.B. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J. Clin. Investig. 1997, 100, 1282. [Google Scholar] [CrossRef]
- Goncalves, A.; Braguer, D.; Kamath, K.; Martello, L.; Briand, C.; Horwitz, S.; Wilson, L.; Jordan, M. Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc. Natl. Acad. Sci. USA 2001, 98, 11737–11742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; O’Brate, A.; Zelnak, A.; Giannakakou, P. Survivin deregulation in β-tubulin mutant ovarian cancer cells underlies their compromised mitotic response to taxol. Cancer Res. 2004, 64, 8708–8714. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marei, H.E.; Casalbore, P.; Althani, A.; Coccè, V.; Cenciarelli, C.; Alessandri, G.; Brini, A.T.; Parati, E.; Bondiolotti, G.; Pessina, A. Human Olfactory Bulb Neural Stem Cells (Hu-OBNSCs) Can Be Loaded with Paclitaxel and Used to Inhibit Glioblastoma Cell Growth. Pharmaceutics 2019, 11, 45. https://doi.org/10.3390/pharmaceutics11010045
Marei HE, Casalbore P, Althani A, Coccè V, Cenciarelli C, Alessandri G, Brini AT, Parati E, Bondiolotti G, Pessina A. Human Olfactory Bulb Neural Stem Cells (Hu-OBNSCs) Can Be Loaded with Paclitaxel and Used to Inhibit Glioblastoma Cell Growth. Pharmaceutics. 2019; 11(1):45. https://doi.org/10.3390/pharmaceutics11010045
Chicago/Turabian StyleMarei, Hany E., Patrizia Casalbore, Asmaa Althani, Valentina Coccè, Carlo Cenciarelli, Giulio Alessandri, Anna T. Brini, Eugenio Parati, Gianpietro Bondiolotti, and Augusto Pessina. 2019. "Human Olfactory Bulb Neural Stem Cells (Hu-OBNSCs) Can Be Loaded with Paclitaxel and Used to Inhibit Glioblastoma Cell Growth" Pharmaceutics 11, no. 1: 45. https://doi.org/10.3390/pharmaceutics11010045
APA StyleMarei, H. E., Casalbore, P., Althani, A., Coccè, V., Cenciarelli, C., Alessandri, G., Brini, A. T., Parati, E., Bondiolotti, G., & Pessina, A. (2019). Human Olfactory Bulb Neural Stem Cells (Hu-OBNSCs) Can Be Loaded with Paclitaxel and Used to Inhibit Glioblastoma Cell Growth. Pharmaceutics, 11(1), 45. https://doi.org/10.3390/pharmaceutics11010045