1. Introduction
Bladder cancer is the most common malignancy of the urinary tract and results from the uncontrolled growth of cells that line the bladder wall. Bladder cancer is common in both men and women, with approximately 75% of patients presenting non-muscle invasive bladder cancer at the time of diagnosis [
1]. Although this type of bladder cancer can be surgically treated by transurethral resection of the tumor, enabling a high survival rate (88–98%), extensive follow-up is still required as surgical resection is associated with a high recurrence rate of up to 70% [
2]. The standard therapy for bladder cancer is a combination of transurethral resection and intravesical instillation of chemotherapeutic agents or immunotherapy with bacillus Calmette–Guérin (BCG), a live attenuated substrain of
Mycobacterium bovis.
Following its first use by Morales et al. in 1976 [
3], BCG has served as the first-line and most effective treatment for bladder cancer. Despite its unique characteristic as cancer immunotherapy that is available in the clinic, BCG has some limitations; it causes severe adverse effects such as BCG abscess, sepsis, and mild cystitis, which affect up to 90% of patients. Furthermore, approximately one-fifth of patients cannot tolerate this therapy [
3]. A recombinant BCG has, thus, been developed to reduce the side effects of BCG and induce a more potent therapeutic effect [
4]. BCG cell wall skeleton (BCG-CWS), a subunit derived from BCG, is an insoluble fraction of the cell wall consisting of mycolic acids and neutral sugars such as arabinose, galactose, and peptidoglycans. BCG-CWS has been introduced as a highly effective immune activator for bladder cancer immunotherapy [
5].
Although the effectiveness of BCG-CWS for cancer immunotherapy has been revealed in several clinical trials [
6], limitations still exist regarding its dosage formulation. BCG-CWS is insoluble in both aqueous and organic solvents and reveals a wide size range of 4.7 to 67.8 μm when dispersed in hydrophilic solvents [
6]. As a result, an oil-in-water emulsion of BCG-CWS is normally used for human application; however, the use of detergents could induce local irritation and/or inflammation [
7]. To efficiently encapsulate BCG-CWS, Nakamura et al. recently derived a unique nanoparticulate technology called liposome evaporated via an emulsified lipid (LEEL) method by using a specific organic solvent that promotes the formation of a nano-sized formulation, with the presence of a condensed structure in the center of the lipid vesicle. With this structure, a compact-shaped of BCG-CWS is established in the center and is covered with lipid bilayers [
6].
The liposomal drug delivery system has been widely used to achieve intracellular delivery of various anti-cancer agents, owing to several advantages, including feasible encapsulation of both hydrophilic and lipophilic drugs and excellent biocompatibility and/or biodegradability [
8]. However, its lack of selectivity to specific cancer cells continues to serve as a barrier for tumor targeting [
9]. To resolve this drawback, liposomes can be surface-modified with different ligands, including peptides, antibodies, small molecules, and carbohydrates [
8]. As folate receptors (FRs) are strongly upregulated in different tumors [
10], folic acid (FA) is an attractive ligand for targeted delivery. Further, for enhanced intracellular delivery, cell-penetrating peptides (CPPs) have been widely applied [
11]. These peptides are relatively short with a net positive charge that enables translocation and successful delivery of various drugs into cells. Pep-1 peptide (Pep1) is one of the most common CPPs with high efficiency of intracellular delivery and a lack of toxicity. Previously, we reported functionalized liposomal systems using these ligands to achieve target cell specificity and improved intracellular uptake of anticancer drug molecules [
12].
To develop a novel nanoparticulate system for the efficient delivery of BCG-CWS, we encapsulated liposomes using a modified LEEL method and performed surface functionalization with FA and Pep1. The intracellular uptake efficiency of BCG-CWS-loaded liposomes was evaluated in FR-expressing cancer cell lines, human-derived 5637, and mouse-derived MBT2 bladder cancer cells. The in vitro immunotherapeutic activity was verified by elevated cytokine production and THP-1 migration assay. Using a xenograft mouse model, the antitumor efficacy was demonstrated via tumor growth inhibition and immune cell infiltration into the tumor tissue. Based on the present study, we propose that FA- and Pep1-modified liposomes encapsulating BCG-CWS might be useful for bladder cancer treatment and may display high target selectivity.
2. Materials and Methods
2.1. Materials
BCG-CWS was sourced from Chungnam National University, Daejeon, Korea. Soy phosphatidylcholine (SPC; purity >99%), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol 2000)] (DSPE-PEG2000-Mal; DP2KM), and distearoylphosphatidylethanolamine-polyethylene glycol5000-folate (DSPE-PEG5000-Fol; DP5KF) were purchased from Avanti® Polar Lipids (Alabaster, AL, USA). 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), poly-l-lysine (PLL), crystal violet, phosphate-buffered saline (PBS) tablets, and cholesterol (CH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). FA was purchased from Duksan Pure Chemical Co., Ltd. (Seoul, Korea). Pep1 (KETWWETWWTEWSQPKKKRKVC, 22mer) was synthesized by Peptron Co. (Daejeon, Korea). Anti-CD4 antibody and anti-interleukin 6 (IL-6) antibody were purchased from Abcam (Cambridge, UK). Acetonitrile, chloroform, methylene chloride, and other solvents purchased from commercial sources were of cell culture or analytical grade. MBT2 and 5637 bladder cancer cell lines were purchased from the Korean Cell Line Bank (Seoul, Korea). Cell culture materials, including fetal bovine serum, Roswell Memorial Institute (RPMI) 1640 medium, trypsin-ethylenediaminetetraacetic acid, and penicillin-streptomycin, and PBS (10×, pH 7.4) were obtained from Invitrogen (Carlsbad, CA, USA). C3H/HeN mice (6–8 weeks, 20 ± 2 g) were purchased from the Hanlim experimental animal laboratory (Gyeonggi-do, Korea).
2.2. Preparation of the Different Liposomal Samples
The following 4 types of BCG-CWS-loaded liposomal nanoparticles were prepared: Plain liposomes (CWS-L), FA-modified liposomes (CWS-FL), Pep1-modified liposomes (CWS-PL), and FA- and Pep1-modified liposomes (CWS-FPL). Based on an earlier report [
6], a slightly modified LEEL method was employed to encapsulate BCG-CWS into the liposomes. Briefly, BCG-CWS (3 mg) was dissolved in methylene chloride (900 μL), and the organic solution was emulsified with PBS solution (2100 μL) containing liposomal vesicles (6.45 mM phospholipid equivalent) using a sonicator (Sonoplus, HD 2070; Bandelin Electronics, Berlin, Germany). After removing the solvent by rotary evaporation, extrusion was performed with a mini-Extruder (Avanti
® Polar Lipids) using 10 passes through a 200 nm membrane filter. DiI, a red fluorescent probe, was co-encapsulated on purpose for visualizing the cell uptake behavior. Empty liposomes were separately prepared by excluding BCG-CWS or DiI. All liposomal samples were stored in a refrigerator (4 °C) and used in experiments within 3 weeks.
2.2.1. CWS-L
The thin film hydration method was used to prepare the plain liposomal vesicles composed of SPC and CH (9:1 molar ratio). Briefly, the components were dissolved in a methanol-chloroform mixture (2:1 v/v), and the organic solvent was rotary evaporated at 40 °C under reduced pressure. The dried thin film was further exposed to a nitrogen gas stream for 2 h and hydrated with 10 mM PBS. The liposomal solution was emulsified with BCG-CWS-containing methylene chloride solution and then extruded as described above.
2.2.2. CWS-FL
To prepare CWS-FL composed of SPC, CH, and DP
5KF (89.5:10:0.5 molar ratio), the post-insertion method was used as reported previously [
13]. Briefly, the prepared CWS-L was incubated with micellized DP
5KF at 60 °C for 1 h. To prepare micelles, the DP
5KF solution (1 mg/mL in chloroform) was rotary evaporated, and the dried film was hydrated with PBS solution. The liposomal solution was emulsified with BCG-CWS-containing methylene chloride solution and extruded as described above. Unincorporated DP
5KF was removed by dialysis for 48 h against distilled water using a cellulose ester dialysis membrane (MWCO 50,000).
2.2.3. CWS-PL
To prepare CWS-PL composed of SPC, CH, DP
2KM, and Pep1 (89.5:10:0.1:0.05 molar ratio), the post-derivatization method was used as reported earlier [
9]. Briefly, maleimide-derivatized liposomes were prepared with DP
2KM and the lipid composition of CWS-L in PBS solution, emulsified with BCG-CWS-containing methylene chloride solution and extruded as described above. Subsequently, the Pep1 solution in PBS was added to modify the vesicle surface via a thiol-maleimide reaction and allowed to react for 12 h. Unconjugated free Pep1 was removed by dialysis, as described above.
2.2.4. CWS-FPL
CWS-FPL, composed of SPC, CH, DP5KF, DP2KM, and Pep1 (89.5:9.9:0.5:0.1:0.05 molar ratio), was prepared by combining post-insertion and post-derivatization methods as described above: A 3-step process by preparing maleimide-derivatized liposomes containing BCG-CWS, followed by Pep1 conjugation and post-insertion of the DP5KF micelle. Purification was performed by dialysis, as described above.
2.3. Particle Size and Zeta Potential (ZP) Analysis
To provide an adequate scattering intensity, liposomal samples were vortexed gently and diluted 1:100 in distilled water before the measurement. Particle size, ZP, and polydispersity index (PDI) were measured in triplicate using a dynamic light scattering particle size analyzer (Zetasizer Nano-ZS; Malvern Instruments, Worcestershire, UK). The particle size of naked BCG-CWS was determined in each sample, and BCG-CWS was dispersed in different organic solvents using a vortex mixer (IKA® vortex GENIUS 3, Staufen, Germany).
2.4. Entrapment Efficiency (EE) and Drug Loading (DL)
The EE and DL of BCG-CWS were determined using a previously reported method [
6]. Briefly, the liposomes were disrupted with ethanol and centrifuged at 3000 g for 5 min under conditions of 15 °C to obtain the BCG-CWS precipitate. The pellet was dissolved in hexane and mixed with a 0.55% carbol-fuchsin solution. The hexane fraction was then collected, and absorbance at 530 nm was measured using a microplate reader (FlexStation 3; Molecular Devices, Sunnyvale, CA, USA). Liposomal samples were subjected to ultra-filtration using Amicon
® ultra-centrifugal filters (MWCO 100,000). To determine the concentration of DiI in the filtrate, an aliquot of the suspension was added to the sample reservoir and centrifuged (14,000 g; 15 °C) for 15 min. For the quantitative determination of DiI, a high-performance liquid chromatography (HPLC) system (Waters
® Corporation; Milford, MA, USA) equipped with separation modules (Waters
® e2695, Waters
® Corporation, Milford, MA, USA), a data station (Empower
® 3, Waters
® Corporation, Milford, MA, USA), and a fluorescence detector (Waters
® W2475, Waters
® Corporation, Milford, MA, USA) was used. Using a mobile phase consisted of 0.05 M dimethyl sulfate and methanol (2:98,
v/v), the chromatography was carried out on a C18 Column (Kromasil
®, 5 μm, 4.6 × 250 mm; Akzo Nobel, Bohus, Sweden) with the excitation and emission wavelengths of 549 and 565 nm, respectively. The EE and DL were calculated using the following Equations (1) and (2):
where
WT,
WF, and WL represent the total amount of the drug (BCG-CWS or DiI) added, the amount of free drug, and the total amount of lipid initially added, respectively.
2.5. Conformational Characterization of Ligand Modification
The extent of ligand modification was determined by HPLC assay using a previously reported method [
9,
14]. Briefly, in the case of the FA ligand, CWS-FL and CWS-FPL were disrupted with 10% Triton X-100, and the content of DP
5KF was determined using a mobile phase consisting of methanol and 10 mM sodium phosphate buffer (pH 7.0; 92:8,
v/v) with a C18 column. The eluent was monitored at 285 nm, and the peak for DP
5KF was separated with the retention time of 2.1 min. Separately, the amount of Pep1 ligand was indirectly quantified by determining the amount of unconjugated Pep1 by HPLC, using a C18 column and a mobile phase consisting of 0.05% trifluoroacetic acid in water (eluent A) and 0.05% trifluoroacetic acid in acetonitrile (eluent B). The eluent gradient ramped from 10% to 60% B in 50 min and subsequently back to 10% B over 5 min. The eluent was monitored at 220 nm, and the peak for Pep1 was separated with the retention time of 32 min.
2.6. Transmission Electron Microscopy (TEM)
For morphology examination, liposomes were imaged using a transmission electron microscope (JEM1010; JEOL, Tokyo, Japan) at an acceleration voltage of 80 kV. Briefly, liposomal samples were diluted 100-fold with distilled water and placed on a carbon film grid. The samples were stained with 2% phosphotungstic acid, washed twice with distilled water, and dried at 25 °C prior to observation.
2.7. Cell Culture
The 5637 and MBT2 bladder cancer cell lines were incubated in a humidified CO2 chamber (Thermo Scientific, Waltham, MA, USA) using RPMI 1640 medium supplemented with penicillin/streptomycin and 10% fetal bovine serum (GIBCO-BRL, Gaithersburg, MD, USA). The cells were sub-cultured every 3–5 days, and cells at passages of 5–20 were used for the experiments.
2.8. Flow Cytometry
Intracellular uptake of the liposomes was determined by measuring the mean fluorescence intensity (MFI) of DiI in the liposome by flow cytometry (FACSCalibur; Becton Dickinson, Franklin Lakes, NJ, USA). Briefly, cells were seeded at a density of 3 × 10
5 cells per well in a 6-well plate with culture media. After 24 h of incubation, the cells were washed twice with PBS and incubated with culture media containing different DiI-loaded liposomes (DiI concentration equivalent to 100 ng/mL). Subsequently, the cells were washed twice with PBS to remove traces of liposomal vesicles remaining within the wells, detached using trypsin-ethylenediaminetetraacetic acid, and suspended in 1 mL of PBS. The suspended cells were introduced into a flow cytometer equipped with a 488 nm argon ion laser. A total of 1 × 10
4 designated cells were collected to quantify the MFI value. To examine the role of FR binding on liposomal uptake, a competitive binding assay was performed with CWS-FL and CWS-FPL, as previously reported [
9,
15]. Briefly, 1 mM of free folic acid was added to the culture media 1 h before treatment. After incubation for 2 h at 37 °C, cells were washed twice with PBS to remove unbound liposomes and excess folic acid. The following steps were performed using the same procedure described above. To prove that the positively-charged Pep1 enhances intracellular delivery, PLL (800 μg/mL), an electrostatic interaction inhibitor [
16], was pre-incubated for 30 min.
2.9. Confocal Laser Scanning Microscopy (CLSM)
To observe the intracellular uptake behavior of liposomes, 5 × 104 cells were seeded in chambered glass slides (Thermo Scientific Nunc, Rochester, NY, USA) and incubated for 24 h at 37 °C, then washed twice with PBS, replenished with fresh culture medium containing the various liposomes, with a DiI concentration of 100 ng/mL. Following incubation for 30 min or 2 h, the cells were washed twice with PBS and fixed with 4% formaldehyde in PBS for 15 min at room temperature. To stain the nuclei and avoid fading, the cells were mounted using Vectashield mounting medium containing DAPI (H-1200). Finally, the cells were observed using a confocal laser scanning microscope (Zeiss LSM 700 Meta; Carl Zeiss Meditec AG, Jena, Germany) under 400× magnification.
2.10. Immunoactivity of CWS-Loaded Liposomes
2.10.1. THP-1 Migration
The 5637 cells were seeded in 24-well plates at a density of 5 × 104 cells per well in a culture medium. After 24 h of incubation, cells were treated with different liposomal formulations for 8 h. To the upper chamber of the transwell (Corning, NY, USA), THP-1 cells in serum-free medium (3 × 105) were added and further incubated for 2 h. The cells on the plate and in the lower chamber were subjected to fixation with 4% paraformaldehyde (Biosaesang, Inc., Gyeonggi-do, Korea) and stained with 0.1% crystal violet. Positive THP-1 staining was visualized by an Olympus CKX41 inverted microscope (Olympus, Tokyo, Japan). Three equal-sized fields were randomly selected for THP-1 cell counting, and an average was calculated.
2.10.2. Cytokine Release
The 5637 cells were seeded into a 60-mm cell culture dish at a density of 1×106 cells. After a 24 h incubation, cells were replenished with fresh medium containing the different liposomal formulations. After 72 h of incubation, the culture medium was collected and centrifuged at a speed of 3000 rpm for 10 min at 4 °C. Samples were either immediately analyzed or aliquoted and stored at −80 °C until analysis. Following the manufacturer’s instructions, the absorbance was measured at 540 nm using a microplate reader (FlexStation 3; Molecular Devices, Sunnyvale, CA, USA).
2.11. In Vivo Antitumor Efficacy
The animal experiment was approved by the Institutional Animal Care and Use Committee of Chung-Ang University (2019-00061, date: 17 June 2019, Seoul, Korea) and carried out in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals. Female C3H/HeN mice were randomly divided into 5 groups (
n = 7): Treatment with the empty liposome (control), CWS-L, CWS-FL, CWS-PL, and CWS-FPL. All mice were subcutaneously inoculated with a mixture of 3.5 × 10
6 MBT2 cells and BCG-CWS-loaded liposomal formulations (equivalent to 0.1 mg of CWS) via a 21G needle injected into their right flank, except mice in the control group, which were inoculated with a mixture of cells and empty liposomes. A digital caliper (Mitutoyo, Kawasaki, Japan) was used to measure the tumor growth periodically, and tumor volume (mm
3) was calculated by the formula: (major axis × minor axis
2) × 0.52 [
6]. The change in tumor volume and body weight of each mouse was observed twice per week for 4 weeks. General animal health and potential side effects were monitored in the aspects of impaired movement, behavioral changes, and food or water avoidance. Mice were sacrificed by cervical dislocation at the end of the experiment, and their tumors were excised and weighed. Median survival time was calculated, and Kaplan-Meier survival curves were plotted using GraphPad Prism (GraphPad Software, San Diego, CA, USA). For immunohistochemistry (IHC) analysis, tumors were further fixed with 4% paraformaldehyde. After embedding in OCT compound (Tissue-Tek
®, Naperville, IL, USA), 3 μm tissue sections were prepared using a cryocut microtome (Leica, Nussloch, Germany).
2.12. Statistical Analysis
All values were expressed as the mean ± standard deviation (SD) (n ≥ 3). Statistical significance was determined using the Student’s t-test, and differences were considered significant at p < 0.05.
4. Discussion
After the antitumor activity of BCG-CWS was revealed, many studies, over time, employed this substitute as cancer immunotherapy [
23,
24,
25]. The main immune activator of BCG is derived from its CWS, which elicits an immune response [
26]. However, its large size and insolubility in aqueous media serve as drawbacks to its use. BCG-CWS is a very large molecule that contains mycolic acid-arabinogalactan-peptidoglycan. As BCG-CWS must be internalized by the bladder cancer cells to initiate the anti-tumor effect, a suitable formulation is a pre-requisite for its use in bladder cancer treatment. In a previous study, liposomal encapsulation of BCG-CWS was attempted using a conventional hydration method; however, aggregation occurred, which exceeded 1 µm. This aggregation was mainly attributed to the hydrophobic interaction among the mycoloyl moieties [
26]. Alternatively, by employing an appropriate organic solvent of pentane, Nakamura et al. developed a “LEEL” method, where the solvent containing BCG-CWS was emulsified with a liposomal solution, followed by solvent evaporation and extrusion. During this encapsulation, BCG-CWS in the hydrophobic solvents becomes smaller and more compact via the formation of a multilayered rolled sheet by inward rolling of its hydrophilic peptidoglycan layer [
27], thereby encapsulating BCG-CWS using lipids to hide it from the hydrophilic environment.
The choice of a suitable solvent for BCG-CWS solvation is a critical factor for nano-sized liposomal encapsulation. In a previous report by Nakamura et al. [
6], pentane was chosen for BCG-CWS dispersion, resulting in a compact size of 96 ± 1 nm. However, in our preliminary study, the use of pentane raised a problem: Size distribution was not uniform and even fluctuated as time passed, which might be attributed to the volatile property of pentane (boiling point 36.1 °C). Therefore, to find an alternative, the size distribution of BCG-CWS in different solvents was measured (
Table S2). As methylene chloride had the smallest and most uniform size and displayed excellent reproducibility among the solvents screened, it was selected as the optimal solvent. The dipole moment of methylene chloride is 1.60, while that of the other solvents is higher, indicating that the size of BCG-CWS depends on the polarity of the solvent used. The preparation of liposomes involved an oil-in-water emulsification and solvent evaporation steps, where the phospholipid functioned as an amphipathic emulsifier. When the solvent was evaporated, BCG-CWS was encapsulated in its hydrophobic form into the liposomes [
6]. However, this type of conventional liposome has a limitation for drug targeting owing to its lack of cell specificity. Unfortunately, to date, there are no trials where bladder cancer cells have been targeted using functionalized liposomes encapsulating BCG-CWS.
For decades, CPPs have been widely used for enhanced intracellular drug delivery [
28]. In a previous study, we introduced Pep1 into liposomes, which remarkably facilitated the translocation of macromolecules, and FITC-dextran encapsulated liposomes [
9]. CPPs might be able to solve the issues related to insufficient intracellular drug delivery. However, because these CPPs are non-specific in their targeting of cancer cells, their application has been limited. To overcome this obstacle, introducing a targeting ligand would be preferred. Among the various types of ligands for surface modification, FA has been extensively used to target FR, which is restrictively expressed in normal cells but highly expressed in various malignant tumors of epithelial origin [
29]. Previously, we established a selective and enhanced drug delivery system using FA-tethered and Pep1-modified liposomes [
9]. Here, different lengths of PEG linker for ligand modification were used: PEG5000 for FA and PEG2000 for Pep1. With this strategy, the FA ligand can orient the outermost layer, thus efficiently targets FR without steric hindrance. Consequently, the number of ligands was optimized with approximately 700 FA molecules and 110 Pep1 molecules. This was achieved via the addition of folate and peptide at respective ratios of 0.5 and 0.05 mol to the liposomes composed of SPC and CH. Based on this optimized liposomal system, we constructed single ligand-modified liposomes (CWS-FL and CWS-PL) and dual ligand-modified liposomes (CWS-FPL) using FA and/or Pep1 to increase the translocation of BCG-CWS-encapsulating liposomes to bladder cancer cells with specific selectivity.
Physical characteristics such as size, drug entrapment, ZP, and colloidal dispersion stability are crucial for nanocarrier formulation. In the present study, all prepared liposomes were less than 200 nm, revealing no influence of ligand modification on vesicular size. Earlier, it was found that FA or Pep1 modification at low molar ratio did not alter the size of liposomes, because the hydrodynamic diameter of the particles could be mainly dominated by the long PEG chains [
9]. With this nano-size, liposomal carriers can readily translocate into the tumoral tissues [
30,
31]. BCG-CWS was encapsulated into the liposomes, with EE values ranging from 59% to 62%. As these values were similar to those in a previous study [
6], the liposomes were deemed to be successfully prepared for BCG-CWS encapsulation. The colloidal stability of the liposomes in the PBS solution was maintained for 3 weeks; thereafter, no signs of aggregation were revealed. Based on ZP, CWS-FL had a higher negative charge than CWS-L, which might be attributed to the presence of negatively charged functional groups derived from the DP
5KF anchored on the liposome surface [
19]. For CWS-PL, the ZP shifted toward a positive charge, which was clearly derived from the positively-charged Pep1. Conversely, CWS-FPL displayed a net negative charge, which might be owing to the difference in PEG chain length: The DP
5KF anchored with a longer PEG covers the Pep1 ligand anchored via DP
2KM with a shorter PEG chain.
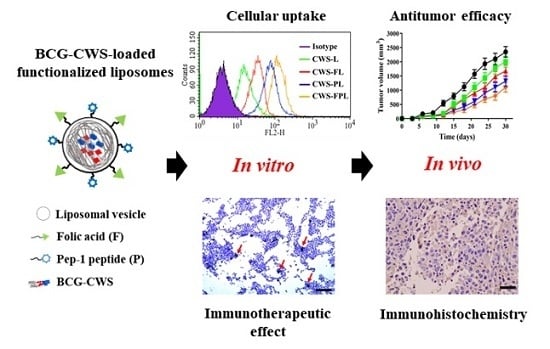
To achieve a sufficient antitumor effect, BCG-CWS should be translocated into the target cancer cell lines. Therefore, in vitro cellular uptake was carried out to determine the efficiency of liposome internalization in 5637 and MBT2 bladder cancer cells. As shown in
Figure 2, the cellular uptake efficiencies of the functionalized liposomes were remarkably higher than that of CWS-L, demonstrating that cellular uptake can be facilitated using the ligands FA and Pep1. Although dual ligand-modified liposomes, such as CWS-FPL, are expected to exhibit enhanced cellular uptake owing to their synergistic effect, their internalization was not drastically higher than that of CWS-FL and CWS-PL. This behavior might be owing to the steric hindrance of the two ligands, as discussed above. To an extent, this result is consistent with the results of earlier studies where the use of high molecular weight PEG was proposed to impede uptake efficiency [
32,
33]. Nonetheless, these results prove that the ligands on the surface of the liposomes interact with their respective receptors, resulting in enhanced uptake when the intracellular concentration of the therapeutic agents is increased. To further investigate the uptake mechanism of the liposomal nanocarriers for BCG-CWS delivery, a competitive assay was carried out. First, the results of this study owing to FA-pretreatment clearly revealed that the uptake process of CWS-FL and CWS-FPL occurred via receptor-mediated endocytosis. This finding is consistent with numerous results that highlight the efficiency of FR-specific drug targeting. Second, PLL was used to determine the effect of the positively-charged Pep1, which inhibits endocytosis by interacting with the negative charge on the surface of cells [
16]. Such findings imply that PLL-pretreatment significantly suppressed the extent of CWS-PL uptake, suggesting that the translocation ability of the CPP-modified liposomes firmly relies on the existence of Pep1. Based on these results, we suggest that the functionalized liposomes could deliver BCG-CWS into the bladder cancer cells with high selectivity and efficient intracellular translocation, thereby enhancing the immunotherapeutic activity.
The immunoactivity of BCG-CWS was assessed by determining cytokine production and THP-1 cell migration after liposomal treatment. Although the exact mechanism whereby BCG exerts its anti-tumor efficacy remains unclear, many studies have shown that the activation of the immune system and induction of an inflammatory response are involved in the antitumor effect [
34,
35]. The BCG-CWS-mediated antitumor effect has been shown to be similar to that of BCG [
22]. The internalization of BCG is known to induce cytokine production by tumor cells, which can be directly toxic to these cells. In addition, BCG interacts with macrophages, enabling the production of cytokines and presentation of BCG-related antigens to T lymphocytes, ultimately resulting in the direct killing of tumor cells [
35]. As the massive release of cytokines, including IL-6 and IFN-γ, occurs following BCG therapy, we evaluated the production of these cytokines to determine the immunotherapeutic effect of BCG-CWS. Because bladder cancer cells secrete IL-6 in response to BCG in vitro [
36], the obtained results imply that BCG-CWS was successfully translocated into the 5637 cells. IFN-γ is a type of cytokine that plays an important role, including as a direct cytotoxic agent against bladder carcinoma. IFN-γ also stimulates the cytotoxicity of macrophages against bladder cancer cells [
37]. Based on our findings, IFN-γ levels were elevated by liposomal treatment. In addition, the monocyte chemotactic effects in the THP-1 migration assay revealed that liposomes increased the migration of THP-1, thereby indicating immune-related cell recruitment. Taken together, the results demonstrate that BCG-CWS encapsulation into liposomes could maintain its inherent immunotherapeutic effect, despite the remarkable difference between the formulations.
In terms of in vivo antitumor efficacy of BCG-CWS, its internalization is expected to result in immune activation, including CD4 infiltration and cytokine production. All BCG-CWS-loaded liposomes caused tumor growth inhibition; however, the degree of suppression was further differentiated by ligand-modified liposomes. As depicted in
Figure 6, the observed intracellular uptake behavior was dependent on the type of liposomes used, specifically the type of ligand-modification. Although the uptake of naked BCG-CWS is extremely limited, that of CWS-L is relatively poor. Based on the ligand used, however, functionalized liposomes displayed enhanced uptake via the following strategies: CWS-FL selectively binds to the FR, resulting in FR-mediated endocytosis; CWS-PL is internalized into the cytosol via cell membrane perturbation caused by Pep1, and CWS-FPL displays a considerably enhanced uptake that involves the combined effect of FA and Pep1. Once BCG-CWS is released into the cytosol, different cytokines are produced that can be cytotoxic to tumor cells. Simultaneously, immune cells such as CD4 and macrophage could infiltrate the cancer cells, resulting in their death.
Numerous studies have shown the antitumor effect of BCG-CWS, which triggers a cascade of immune responses [
22]. Thus, to better understand its antitumor effect, IL-6 and CD4 were employed. IL-6 is one of the main cytokines released from bladder cancer cells [
38], and it recruits neutrophils to the site of the cancer, which also contributes to its anticancer effects [
34]. The IHC results revealed that the BCG-CWS-loaded liposomes were internalized by bladder cancer cells. This is because the release of IL-6 is known to be dependent on the internalization of BCG, which might be owing to the BCG-CWS used herein. As brown spots were clearly visible, this implied the production of IL-6. Nonetheless, the difference in formulation was further elucidated according to the immunohistochemical scores. CD4, a type of T lymphocyte, is considered to be one of the most effective immune cells for eliminating cancer cells [
39]. In an earlier study, CD4 and CD8 lymphocytes were shown to be essential for successful BCG immunotherapy. This was proven in a murine bladder cancer model where depletion of either CD4 or CD8 lymphocyte resulted in a loss in BCG-mediated antitumor activity [
40]. In the present study, we evaluated CD4 expression using the BCG-CWS-loaded liposomes to determine the correlation between the enhanced antitumor effect and CD4 infiltration. As all liposome-treated groups showed brown spots, the difference between the groups was further visualized through an enlargement of the brown spot region. The IHC scores clearly revealed that enhanced internalization of BCG-CWS-loaded liposomes resulted in the elevation of CD4 infiltration. Altogether, these findings indicate that the encapsulation of BCG-CWS into the functionalized liposomes is beneficial for intracellular translocation of the cargo into bladder cancer cells, ultimately mediating the antitumor effect with IL-6 and CD4.