Localized Therapy of Vaginal Infections and Inflammation: Liposomes-In-Hydrogel Delivery System for Polyphenols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Liposomes
2.3. Characterization of Liposomes
2.3.1. Vesicle Size
2.3.2. Zeta Potential
2.3.3. Polyphenol Entrapment Efficiency
2.4. Preparation of Hydrogels
2.4.1. Chitosan Hydrogels
2.4.2. Preparation of Liposomes-In-Hydrogel Formulation
2.5. Preparation of Vaginal Tissue
2.6. Characterization of Hydrogels
2.6.1. Texture Analysis
2.6.2. Mucoadhesive Properties
2.6.3. In Vitro Polyphenol Release
2.6.4. Preparation of Sheep Vaginal Tissue
2.6.5. Ex Vivo Penetration
2.6.6. Polyphenol Quantification by HPLC
2.7. Cell Toxicity
2.8. Anti-Oxidative Activities
2.8.1. DPPH Radical Scavenging
2.8.2. ABTS·+ Radical Scavenging
2.9. Anti-Inflammatory Activity Measurement
2.10. Statistical Analyses
3. Results and Discussion
3.1. Liposomal Characteristics
3.2. Liposomes-In-Hydrogel Characteristics
3.2.1. Texture and Mucoadhesiveness
3.2.2. Polyphenol Release Studies
3.3. Effect of Polyphenol Liposomes-In-Hydrogel on Cell Toxicity
3.4. Anti-Oxidative Activity of EPI
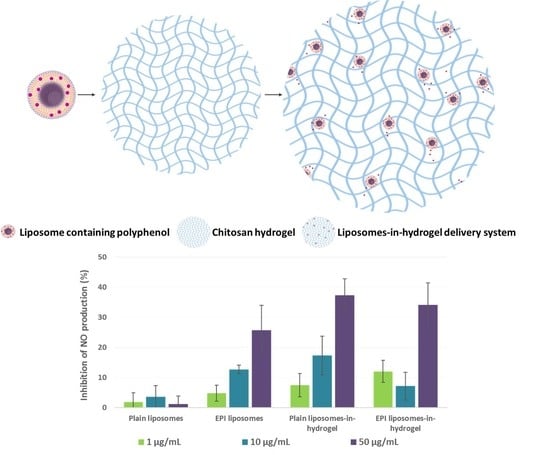
3.5. Anti-Inflammatory Activity of EPI
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2′-azino bis(3-ethylbenzothiazoline)-6-sulfonic acid diammonium salt |
| DPPH | 1-diphenyl-2-picrylhydrazyl |
| EPI | epicatechin |
| LPS | lipopolysaccharide |
| NO | nitric oxide |
| PBS | phosphate buffer saline |
| PC | phosphatidylcholine |
| PI | polydispersity index |
| RES | resveratrol |
| STI | sexually transmitted infections |
References
- De Ambrogi, M. Turning the spotlight on sexually transmitted infections. Lancet Infect. Dis. 2017, 17, 792–793. [Google Scholar] [CrossRef]
- Unemo, M.; Bradshaw, C.S.; Hocking, J.S.; de Vries, H.J.C.; Fransis, S.C.; Mabey, D.; Marrazzo, J.M.; Sonder, G.J.B.; Schwebeke, J.R.; Hoornenborg, E.; et al. Sexually transmitted infections: Challenges ahead. Lancet. Infect. Dis. 2017, 17, e235–e279. [Google Scholar] [CrossRef]
- Basnet, P.; Hussain, H.; Tho, I.; Škalko-Basnet, N. Liposomal delivery system enhances anti-inflammatory properties of curcumin. J. Pharm. Sci. 2012, 101, 598–609. [Google Scholar] [CrossRef]
- Berginc, K.; Škalko-Basnet, N.; Basnet, P.; Kristl, A. Development and evaluation of an in vitro vaginal model for assessment of drug’s biopharmaceutical properties: Curcumin. AAPS PharmSciTech 2012, 13, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Škalko-Basnet, N.; Acharya, G.; Basnet, P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 2015, 79, 112–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Qiao, Z. Analysis of efficacy of resveratrol treatment in patients with scarred uterus. Exp. Ther. Med. 2018, 15, 5410–5414. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Meng, X.-M.; Li, B.; Wang, C.-Q.; Wang, S.-Q.; Wang, T.-L.; Tian, Y.-M. Inhibition of alpha-hemolysin sxpression by resveratrol attenuates Staphylococcus aureus virulence. Microb. Pathog. 2019, 127, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Naveed, M.; Arif, M.; Kakar, M.U.; Manzoor, R.; El-Hack, M.E.A.; Alagawany, M.; Tiwari, R.; Khandia, R.; Munjal, A.; et al. Green tea (Camellia sinensis) and l-theanine: Medicinal values and beneficial applications in humans-A comprehensive review. Biomed. Pharmacother. 2017, 95, 1260–1275. [Google Scholar] [CrossRef]
- Gross, G. Polyphenon E—Eine neue topische Therapie für Condylomata acuminata. Hautarzt 2008, 59, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Yu, Z.; Zhang, X.; Li, W.; Gao, D.; Wang, J.; Ma, X.; Nie, X.; Wang, W. Epicatechin alleviates inflammation in lipopolysaccaride-induced acute lung injury in mice inhibiting the p38 MAPK signaling pathway. Int. Immunopharmacol. 2019, 66, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Škalko-Basnet, N. Nanopharmaceuticals for improved topical vaginal therapy: Can they deliver? Eur. J. Pharm. Sci. 2013, 50, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Pavelić, Ž.; Škalko-Basnet, N.; Schubert, R. Liposomal gels for vaginal drug delivery. Int. J. Pharm. 2001, 219, 139–149. [Google Scholar] [CrossRef]
- Das Neves, J.; Bahia, M. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar] [CrossRef]
- Hussain, A.; Ahsan, F. The vagina as a route for systemic drug delivery. J. Control. Release 2005, 103, 301–313. [Google Scholar] [CrossRef]
- Yu, T.; Malcolm, K.; Woolfson, D.; Jones, D.J.; Andrews, G.P. Vaginal gel drug delivery systems: Understanding rheological characteristics and performance. Expert. Opin. Drug Deliv. 2011, 8, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, J.; Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Rodrigues, F.; Sarmento, B. Vaginal Mucosa and Drug Delivery. In Mucoadhesive Materials and Drug Delivery Systems; Wiley: Chichester, UK, 2014; pp. 99–131. [Google Scholar]
- Perinelli, D.R.; Campana, R.; Skouras, A.; Bonacucina, G.; Cespi, M.; Mastrotto, F.; Baffone, W.; Casettari, L. Chitosan Loaded into a Hydrogel Delivery System as a Strategy to Treat Vaginal Co-Infection. Pharmaceutics 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Valenta, C. The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 2005, 57, 1692–1712. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Vanić, Ž.; Tho, I.; Škalko-Basnet, N. Chitosan-coated liposomes for topical vaginal therapy: Assuring localized drug effect. Int. J. Pharm. 2014, 472, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jøraholmen, M.W.; Basnet, P.; Acharya, G.; Škalko-Basnet, N. PEGylated liposomes for topical vaginal therapy improve delivery of interferon alpha. Eur. J. Pharm. Biopharm. 2017, 113, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Hurler, J.; Škalko-Basnet, N. Potentials of Chitosan-Based Delivery Systems in Wound Therapy: Bioadhesion Study. J. Funct. Biomater. 2012, 3, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Hurler, J.; Engesland, A.; Kermany, B.P.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Kristl, J.; Teskač, K.; Caddeo, C.; Abramović, Z.; Šentjurc, M. Improvements of cellular stress response on resveratrol in liposomes. Eur. J. Pharm. Biopharm. 2009, 73, 253–259. [Google Scholar] [CrossRef]
- Li, D.; Martini, N.; Wu, Z.; Wen, J. Development of an isocratic HPLC method for catechin quantification and its application to formulation studies. Fitoterapia 2012, 83, 1267–1274. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhai, G. Advances in lipid-based colloidal systems as drug carrier for topic delivery. J. Control Release 2014, 193, 90–99. [Google Scholar] [CrossRef]
- Pavelić, Ž.; Škalko-Basnet, N.; Filipović-Grčić, J.; Martinac, A.; Jalšenjak, I. Development and in vitro evaluation of a liposomal vaginal delivery system for acyclovir. J. Control Release 2005, 106, 34–43. [Google Scholar] [CrossRef]
- Das Neves, J.; Bahia, M.F.; Amiji, M.M.; Sarmento, B. Mucoadhesive nanomedicines: Characterization and modulation of mucoadhesion at the nanoscale. Expert Opin Drug Deliv 2011, 8, 1085–1104. [Google Scholar] [CrossRef]
- Ong, S.G.M.; Chitneni, M.; Lee, K.S.; Ming, L.C.; Yuen, K.H. Evaluation of extrusion technique for nanosizing liposomes. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef]
- Soema, P.C.; Willems, G.J.; Jiskoot, W.; Amorij, J.P.; Kersten, G.F. Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur. J. Pharm. Biopharm. 2015, 94, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.Y.; Hung, C.F.; Hwang, T.L.; Huang, Y.L. Physicochemical characteristics and in vivo deposition of liposome-encapsulated tea catechins by topical and intratumor administrations. J. Drug Target 2005, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Žakelj, S.; Mravljak, J.; Pajk, S.; Kristl, A.; Schubert, R.; Škalko-Basnet, N. The effect of lipid composition and liposome size on the release properties of liposomes-in-hydrogel. Int. J. Pharm. 2013, 456, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.; Storm, G. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols–a review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Mignet, N.; Seguin, J.; Chabot, G. Bioavailability of polyphenol liposomes: A challenge ahead. Pharmaceutics 2013, 5, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Teskač, K.; Sinico, C.; Kristl, J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int. J. Pharm. 2008, 363, 183–191. [Google Scholar] [CrossRef]
- Huang, Y.B.; Tsai, M.J.; Wu, P.C.; Tsai, Y.H.; Wu, Y.H.; Fang, J.Y. Elastic liposomes as carriers for oral delivery and the brain distribution of (+)-catechin. J. Drug Target 2011, 19, 709–718. [Google Scholar] [CrossRef]
- Pitorre, M.; Gondé, H.; Haury, C.; Messous, M.; Poilane, J.; Boudaud, D.; Kanber, E.; Ndombina, G.A.R.; Benoit, J.P.; Bastiat, G. Recent advances in nanocarrier-loaded gels: Which drug delivery technologies against which diseases? J. Control Release 2017, 266, 140–155. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Giunchedi, P.; Scalia, S.; Rossi, S.; Sandri, G.; Caramella, C. Chitosan gels for the vaginal delivery of lactic acid: Relevance of formulation parameters to mucoadhesion and release mechanism. AAPS PharmaSciTech 2006, 5, E141–E147. [Google Scholar] [CrossRef]
- Bassi da Silva, J.; Ferreira, S.B.d.S.; de Freitas, O.; Bruschi, M.L. A critical review about methodologies for the analysis of mucoadhesive properties of drug delivery systems. Drug Dev Ind Pharm 2017, 43, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.K.; Friedel, H.D.; Barker, A.R.; Buhse, L.F.; Keitel, S.; Cecil, T.L.; Kraemer, J.; Morris, J.M.; Reppas, C.; Stickelmeyer, M.P. FIP/AAPS joint workshop report: Dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech 2011, 12, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Siewert, M.; Dressman, J.; Brown, C.K.; Shah, V.P.; Aiache, J.M.; Aoyagi, N.; Bashaw, D.; Brown, C.; Brown, W.; Burgess, D. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech 2003, 4, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; González-Manzano, S.; González-Paramás, A.; Santos-Buelga, C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J. Pharm. Biomed. Anal. 2010, 51, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Lucio, M.; Ferreira, H.; Lima, J.F.L.S.; Reis, S. Use of liposomes as membrane models to evaluate the contribution of drug–membrane interactions to antioxidant properties of etodolac. Redox Rep. 2008, 13, 225–236. [Google Scholar] [CrossRef]
- Cook, M.T.; Brown, M.B. Polymeric gels for intravaginal drug delivery. J. Control Release 2017, 270, 145–157. [Google Scholar] [CrossRef]
- Frank, L.A.; Chaves, P.S.; D’amore, C.M.; Contri, R.V.; Frank, A.G.; Beck, R.C.; Pohlmann, A.R.; Buffon, A.; Guterres, S.S. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: Increasing penetration and adhesion of imiquimod in vaginal tissue. Eur. J. Pharm. Biopharm. 2017, 114, 202–212. [Google Scholar] [CrossRef]
- Yoon, H.J.; Moon, M.E.; Park, H.S.; Im, S.Y.; Kim, Y.H. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem. Biophys. Res. Commun. 2007, 358, 954–959. [Google Scholar] [CrossRef]









| Vesicle Size (nm) | PI * | Zeta Potential (mV) | Entrapment (%) | Polyphenol/Lipid Ratio (µg/mg) | |
|---|---|---|---|---|---|
| RES liposomes | 192 ± 15 | 0.100 | −3.42 ± 1.02 | 81 ± 10 | 54.29 ± 2.33 |
| EPI liposomes | 196 ± 13 | 0.072 | −3.32 ± 1.06 | 77 ± 2 | 48.48 ± 3.05 |
| RES Liposomes | EPI Liposomes | |||
|---|---|---|---|---|
| Chitosan concentration (%, w/w) | 2.5 | 3 | 2.5 | 3 |
| Detachment force [g] | 10.66 ± 1.59 | 11.01 ± 1.43 | 9.82 ± 1.39 | 11.88 ± 0.43 |
| Formulation retained on tissue (%) | 73 ± 5 | 72 ± 3 | 79 ± 5 | 70 ± 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jøraholmen, M.W.; Basnet, P.; Tostrup, M.J.; Moueffaq, S.; Škalko-Basnet, N. Localized Therapy of Vaginal Infections and Inflammation: Liposomes-In-Hydrogel Delivery System for Polyphenols. Pharmaceutics 2019, 11, 53. https://doi.org/10.3390/pharmaceutics11020053
Jøraholmen MW, Basnet P, Tostrup MJ, Moueffaq S, Škalko-Basnet N. Localized Therapy of Vaginal Infections and Inflammation: Liposomes-In-Hydrogel Delivery System for Polyphenols. Pharmaceutics. 2019; 11(2):53. https://doi.org/10.3390/pharmaceutics11020053
Chicago/Turabian StyleJøraholmen, May Wenche, Purusotam Basnet, Mia Jonine Tostrup, Sabrin Moueffaq, and Nataša Škalko-Basnet. 2019. "Localized Therapy of Vaginal Infections and Inflammation: Liposomes-In-Hydrogel Delivery System for Polyphenols" Pharmaceutics 11, no. 2: 53. https://doi.org/10.3390/pharmaceutics11020053
APA StyleJøraholmen, M. W., Basnet, P., Tostrup, M. J., Moueffaq, S., & Škalko-Basnet, N. (2019). Localized Therapy of Vaginal Infections and Inflammation: Liposomes-In-Hydrogel Delivery System for Polyphenols. Pharmaceutics, 11(2), 53. https://doi.org/10.3390/pharmaceutics11020053