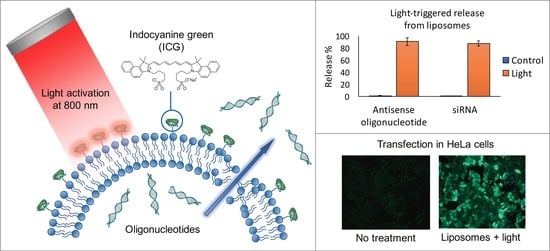
Light-Triggered Cellular Delivery of Oligonucleotides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- SSO: 5′-GCT ATT ACC TTA ACC CAG-3′
- siRNA: sense 5′-CUUACGCUGAGUACUUCGAdTdT-3′
- anti-sense 5′-UCGAAGUACUCAGCGUAAGdTdT-3′
2.2. Liposome Preparation
2.3. Liposome Characterization
2.3.1. Size
2.3.2. Zeta-Potential
2.3.3. Phase Transition Temperature (Tm)
2.3.4. Encapsulation Efficiency
2.3.5. Light-Induced Oligonucleotide Release
2.4. Cell Studies
2.4.1. Cell Line
2.4.2. Cell Culture
2.4.3. Transfection Studies
2.4.4. Cytotoxicity
2.4.5. Image Analysis
3. Results
3.1. Characterization of the Oligonucleotide-Encapsulated ICG Liposomes
3.2. Transfection Experiments
3.2.1. Liposome Concentration
3.2.2. ICG Concentration
3.2.3. Illumination Time
3.2.4. Light Intensity
3.2.5. Free ICG and SSO
3.2.6. Time after Transfection and Light Triggering
3.3. Cytotoxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, C.M.; Harris, E.N. Antisense oligonucleotides: Treatment strategies and cellular internalization. RNA Dis. 2016, 3, e1393. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.; Chopra, D.; Wardha, N. Antisense oligonucleotide: Basic concept and its therapeutic application. J. Res. Pharm. Sci. 2014, 2, 1–13. [Google Scholar]
- Juliano, R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016, 44, 6518–6548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lächelt, U.; Wagner, E. Nucleic Acid Therapeutics Using Polyplexes: A Journey of 50 Years (and Beyond). Chem. Rev. 2015, 115, 11043–11078. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, L.; Satterlee, A.; Huang, L. Delivery of oligonucleotides with lipid nanoparticles. Adv. Drug Deliv. Rev. 2015, 87, 68–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jesus, M.B.; Zuhorn, I.S. Solid lipid nanoparticles as nucleic acid delivery system: Properties and molecular mechanisms. J. Control. Release 2015, 201, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, R.; Eloy, J.O.; Marchetti, J.M.; Lopez, R.F.; Lee, R.J. Targeted lipid nanoparticles for antisense oligonucleotide delivery. Curr. Pharm. Biotechnol. 2014, 15, 847–855. [Google Scholar] [CrossRef]
- Audouy, S.A.; de Leij, L.F.; Hoekstra, D.; Molema, G. In vivo characteristics of cationic liposomes as delivery vectors for gene therapy. Pharm. Res. 2002, 19, 1599–1605. [Google Scholar] [CrossRef]
- Knudsen, K.B.; Northeved, H.; Kumar, P.E.; Permin, A.; Gjetting, T.; Andresen, T.L.; Larsen, S.; Wegener, K.M.; Lykkesfeldt, J.; Jantzen, K.; et al. In vivo toxicity of cationic micelles and liposomes. Nanomedicine 2015, 11, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Zelphati, O.; Uyechi, L.S.; Barron, L.G.; Szoka, F.C., Jr. Effect of serum components on the physico-chemical properties of cationic lipid/oligonucleotide complexes and on their interactions with cells. Biochim. Biophys. Acta 1998, 1390, 119–133. [Google Scholar] [CrossRef]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. 2012, 51, 8529–8533. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.J.W.; Kulkarni, J.A.; van der Meel, R.; Cullis, P.R.; Vader, P.; Schiffelers, R.M. State-of-the-art design and rapid-mixing production techniques of lipid nanoparticles for nucleic acid delivery. Small Methods 2018, 2, 1700375. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, F.; Hu, R.G.; Becker, D.L.; Xu, C. Stimuli-responsive liposomes for the delivery of nucleic acid therapeutics. Nanomedicine 2015, 11, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Lajunen, T.; Kontturi, L.S.; Viitala, L.; Manna, M.; Cramariuc, O.; Róg, T.; Bunker, A.; Laaksonen, T.; Viitala, T.; Murtomäki, L.; et al. Indocyanine Green-Loaded Liposomes for Light-Triggered Drug Release. Mol. Pharm. 2016, 13, 2095–2107. [Google Scholar] [CrossRef]
- Lajunen, T.; Nurmi, R.; Kontturi, L.S.; Viitala, L.; Yliperttula, M.; Murtomäki, L.; Urtti, A. Light activated liposomes: Functionality and prospects in ocular drug delivery. J. Control. Release 2016, 244, 157–166. [Google Scholar] [CrossRef]
- Viitala, L.; Pajari, S.; Lajunen, T.; Kontturi, L.S.; Laaksonen, T.; Kuosmanen, P.; Viitala, T.; Urtti, A.; Murtomäki, L. Photothermally triggered lipid bilayer phase transition and drug release from gold nanorod and indocyanine green encapsulated liposomes. Langmuir 2016, 32, 4554–4563. [Google Scholar] [CrossRef]
- Desmettre, T.; Devoisselle, J.; Mordon, S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv. Ophthalmol. 2000, 45, 15–27. [Google Scholar] [CrossRef]
- Yannuzzi, L.A. Indocyanine green angiography: A perspective on use in the clinical setting. Am. J. Ophthalmol. 2011, 151, 745–751. [Google Scholar] [CrossRef]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed]
- Eichler, J.; Knof, J.; Lenz, H. Measurements on the depth of penetration of light (0.35–1.0 μm) in tissue. Radiat. Environ. Biophys. 1977, 14, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, C.; Roza, L.; Epe, B. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis 1997, 18, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Tse, B.W.C.; Yang, H.; Thorling, C.A.; Liu, Y.; Touraud, M.; Chouane, J.B.; Liu, X.; Roberts, M.S.; et al. Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics 2018, 8, 1227–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Liang, X.; Xu, Y.; Yang, Y.; Li, X.; Dai, Z. Doxorubicin and indocyanine green loaded hybrid bicelles for fluorescence imaging guided synergetic chemo/photothermal therapy. Bioconjug. Chem. 2017, 28, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Si, T.; Xu, R.X. Microencapsulation of indocyanine green for potential applications in image-guided drug delivery. Lab Chip 2015, 15, 646–649. [Google Scholar] [CrossRef] [Green Version]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. How toxic are gold nanoparticles? The state-of-the-art. Nano Res. 2015, 8, 1771. [Google Scholar] [CrossRef]
- Lee, K.; Jeong, J.; Choy, J.H. Toxicity evaluation of inorganic nanoparticles: Considerations and challenges. Mol. Cell. Toxicol. 2013, 9, 205–210. [Google Scholar]
- Sazani, P.; Kang, S.H.; Maier, M.A.; Wei, C.; Dillman, J.; Summerton, J.; Manoharan, M.; Kole, R. Nuclear antisense effects of neutral, anionic and cationic oligonucleotide analogs. Nucleic Acids Res. 2001, 29, 3965–3974. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.H.; Cho, M.J.; Kole, R. Up-regulation of luciferase gene expression with antisense oligonucleotides: Implications and applications in functional assay development. Biochemistry 1998, 37, 6235–6239. [Google Scholar] [CrossRef]
- Lajunen, T.; Nurmi, R.; Wilbie, D.; Ruoslahti, T.; Johansson, N.G.; Korhonen, O.; Rog, T.; Bunker, A.; Ruponen, M.; Urtti, A. The effect of light sensitizer localization on the stability of indocyanine green liposomes. J. Control. Release 2018, 284, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zelphati, O.; Szoka, F.C. Mechanism of oligonucleotide release from cationic liposomes. Proc. Natl. Acad. Sci. USA 1996, 93, 11493–11498. [Google Scholar] [CrossRef] [PubMed]
- Ruozi, B.; Battini, R.; Tosi, G.; Forni, F.; Vandelli, M.A. Liposome-oligonucleotides interaction for in vitro uptake by COS I and HaCaT cells. J. Drug Target 2005, 13, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.P.; Berezhna, S.; Scherfeld, D.; Kahya, N.; Schwille, P. Characterization of interaction between cationic lipid-oligonucleotide complexes and cellular membrane lipids using confocal imaging and fluorescence correlation spectroscopy. Biophys. J. 2005, 88, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhuang, S.; Qi, X.R. Comparative study of the in vitro and in vivo characteristics of cationic and neutral liposomes. Int. J. Nanomed. 2011, 6, 3087–3098. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef]
- Miller, C.R.; Bondurant, B.; McLean, S.D.; McGovern, K.A.; O’Brien, D.F. Liposome-cell interactions in vitro: Effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry 1998, 37, 12875–12883. [Google Scholar] [CrossRef]
- Xia, Y.; Tian, J.; Chen, X. Effect of Surface Properties on Liposomal siRNA Delivery. Biomaterials 2016, 79, 56–68. [Google Scholar] [CrossRef]
- Kraft, J.C.; Ho, R.J. Interactions of indocyanine green and lipid in enhancing near-infrared fluorescence properties: The basis for near-infrared imaging in vivo. Biochemistry 2014, 53, 1275–1283. [Google Scholar] [CrossRef]
- Kim, T.H.; Chen, Y.; Mount, C.W.; Gombotz, W.R.; Li, X.; Pun, S.H. Evaluation of temperature-sensitive, indocyanine green-encapsulating micelles for noninvasive near-infrared tumor imaging. Pharm. Res. 2010, 27, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Moyle, P.M.; Toth, I. Endosome escape strategies for improving the efficacy of oligonucleotide delivery systems. Curr. Med. Chem. 2015, 22, 3326–3346. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Carver, K. Cellular uptake and intracellular trafficking of oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelphati, O.; Szoka, F.C., Jr. Intracellular distribution and mechanism of delivery of oligonucleotides mediated by cationic lipids. Pharm. Res. 1996, 13, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Paasonen, L.; Sipilä, T.; Subrizi, A.; Laurinmäki, P.; Butcher, S.J.; Rappolt, M.; Yaghmur, A.; Urtti, A.; Yliperttula, M. Gold-embedded photosensitive liposomes for drug delivery: Triggering mechanism and intracellular release. J. Control. Release 2010, 147, 136–143. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Urtti, A. Current and future ophthalmic drug delivery systems: A shift to the posterior segment. Drug Discov. Today 2008, 13, 135–143. [Google Scholar] [CrossRef]
- Christie, J.G.; Kompella, U.B. Ophthalmic light sensitive nanocarrier systems. Drug Discov. Today 2008, 13, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Martens, T.F.; Vercauteren, D.; Forier, K.; Deschout, H.; Remaut, K.; Paesen, R.; Ameloot, M.; Engbersen, J.F.; Demeester, J.; De Smedt, S.C.; et al. Measuring the intravitreal mobility of nanomedicines with single-particle tracking microscopy. Nanomedicine 2013, 8, 1955–1968. [Google Scholar] [CrossRef] [PubMed]







| Liposome Type | ICG Concentration | Diameter ± SD (nm) | PDI | Encapsulation % ± SD |
|---|---|---|---|---|
| SSO liposomes | without ICG | 145 ± 3 | 0.123 | 6.7 ± 0.9 |
| with 1/25 ICG/lipid | 150 ± 1 | 0.102 | ||
| siRNA liposomes | without ICG | 153 ± 2 | 0.141 | 5.8 ± 0.7 |
| with 1/25 ICG/lipid | 155 ± 2 | 0.116 |
| Liposome Type | ICG Concentration | Zeta-Potential ± SD (mV) | Tm (°C) |
|---|---|---|---|
| SSO liposomes | without ICG | −8.1 ± 0.1 | 42.7 |
| with 1/50 ICG/lipid | −12.9 ± 0.3 | 42.1 | |
| with 1/25 ICG/lipid | −16.4 ± 0.3 | 41.9 | |
| siRNA liposomes | without ICG | −8.1 ± 0.3 | 42.6 |
| with 1/50 ICG/lipid | −12.2 ± 0.3 | 42.0 | |
| with 1/25 ICG/lipid | −16.5 ± 0.3 | 42.1 | |
| Empty liposomes | without ICG | −8.2 ± 0.1 | 42.7 |
| with 1/50 ICG/lipid | −11.9 ± 0.4 | 42.3 | |
| with 1/25 ICG/lipid | −15.9 ± 0.2 | 42.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontturi, L.-S.; van den Dikkenberg, J.; Urtti, A.; Hennink, W.E.; Mastrobattista, E. Light-Triggered Cellular Delivery of Oligonucleotides. Pharmaceutics 2019, 11, 90. https://doi.org/10.3390/pharmaceutics11020090
Kontturi L-S, van den Dikkenberg J, Urtti A, Hennink WE, Mastrobattista E. Light-Triggered Cellular Delivery of Oligonucleotides. Pharmaceutics. 2019; 11(2):90. https://doi.org/10.3390/pharmaceutics11020090
Chicago/Turabian StyleKontturi, Leena-Stiina, Joep van den Dikkenberg, Arto Urtti, Wim E. Hennink, and Enrico Mastrobattista. 2019. "Light-Triggered Cellular Delivery of Oligonucleotides" Pharmaceutics 11, no. 2: 90. https://doi.org/10.3390/pharmaceutics11020090
APA StyleKontturi, L. -S., van den Dikkenberg, J., Urtti, A., Hennink, W. E., & Mastrobattista, E. (2019). Light-Triggered Cellular Delivery of Oligonucleotides. Pharmaceutics, 11(2), 90. https://doi.org/10.3390/pharmaceutics11020090