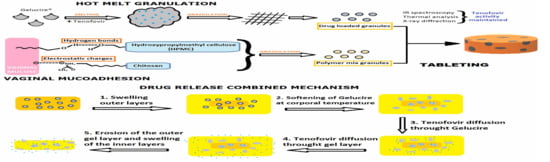
Tenofovir Hot-Melt Granulation using Gelucire® to Develop Sustained-Release Vaginal Systems for Weekly Protection against Sexual Transmission of HIV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Granules
2.3. Characterization of the Granules
2.3.1. Infrared Spectroscopy
2.3.2. Thermal Analysis
2.3.3. X-ray Diffraction
2.4. Preparation of the Tablets
2.5. Assessment of the Tablets
2.5.1. Swelling Behavior
2.5.2. Release Study
2.5.3. Mucoadhesion Test
3. Results and Discussion
3.1. Characterization of the Granules
3.1.1. Infrared Spectroscopy
3.1.2. Thermal Analysis
3.1.3. X-ray Diffraction
3.2. Assessment of the Tablets
3.2.1. Swelling Behavior
3.2.2. Release Study
3.2.3. Mucoadhesion Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaudhary, R.S.; Amankwaa, E.; Kumar, S.; Hu, T.; Chan, M.; Sanghvi, P. Application of a hot-melt granulation process to enhance fenofibrate solid dose manufacturing. Drug Develop. Ind. Pharm. 2015, 42, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Jakson, K.A.; Hunt, J.D. Binary eutectic solidification. Trans Met Soc AIME 1966, 236, 843–852. [Google Scholar]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2015, 17, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Ho, H.-O.; Chiou, J.-D.; Sheu, M.-T. Physical and dissolution characterization of cilostazol solid dispersions prepared by hot melt granulation (HMG) and thermal adhesion granulation (TAG) methods. Int. J. Pharm. 2014, 473, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, E.; De Beer, T.; Mooter, G.V.D.; Remon, J.; Vervaet, C. Influence of formulation and process parameters on the release characteristics of ethylcellulose sustained-release mini-matrices produced by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2008, 69, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Özgüney, I.; Shuwisitkul, D.; Bodmeier, R. Development and characterization of extended release Kollidon® SR mini-matrices prepared by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2009, 73, 140–145. [Google Scholar] [CrossRef]
- Verstraete, G.; Mertens, P.; Grymonpré, W.; Van Bockstal, P.-J.; De Beer, T.; Boone, M.N.; Van Hoorebeke, L.; Remon, J.; Vervaet, C. A comparative study between melt granulation/compression and hot melt extrusion/injection molding for the manufacturing of oral sustained release thermoplastic polyurethane matrices. Int. J. Pharm. 2016, 513, 602–611. [Google Scholar] [CrossRef]
- Almeida, A.; Saerens, L.; De Beer, T.; Remon, J.; Vervaet, C. Upscaling and in-line process monitoring via spectroscopic techniques of ethylene vinyl acetate hot-melt extruded formulations. Int. J. Pharm. 2012, 439, 223–229. [Google Scholar] [CrossRef]
- El Hadri, M.; Achahbar, A.; El Khamkhami, J.; Khelifa, B.; Faivre, V.; Abbas, O.; Bresson, S. Lyotropic behavior of Gelucire 50/13 by XRD, Raman and IR spectroscopies according to hydration. Chem. Phys. Lipids 2016, 200, 11–23. [Google Scholar] [CrossRef]
- Chambin, O.; Jannin, V. Interest of Multifunctional Lipid Excipients: Case of Gelucire® 44/14. Drug Develop. Ind. Pharm. 2005, 31, 527–534. [Google Scholar] [CrossRef]
- Upadhyay, P.; Pandit, J.K.; Wahi, A.K. Gelucire: An alternative formulation technological tool for both sustained and fast release of drugs in treating diabetes mellitus type II disease. J. Sci. Ind. Res. 2013, 72, 776–780. [Google Scholar]
- UNAIDS. When Women Lead Change Happens. Available online: http://www.unaids.org/sites/default/files/media_asset/when-women-lead-change-happens_en.pdf (accessed on 30 December 2017).
- Aurpibul, L.; Puthanakit, T. Review of Tenofovir Use in HIV-infected Children. Pediatric Infect. Dis. J. 2015, 34, 383–391. [Google Scholar] [CrossRef]
- Mertenskoetter, T.; Kaptur, P. Update on microbicide research and development-seeking new HIV prevention tools for women. Eur. J. Med Res. 2011, 16, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hankins, C.A.; Dybul, M.R. The promise of pre-exposure prophylaxis with antiretroviral drugs to prevent HIV transmission: A review. Curr. Opin. HIV AIDS 2013, 8, 50–58. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.-D. Optimization of tenofovir release from mucoadhesive vaginal tablets by polymer combination to prevent sexual transmission of HIV. Carbohydr. Polym. 2018, 179, 305–316. [Google Scholar] [CrossRef]
- Cazorla-Luna, R.; Notario-Pérez, F.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.D. Chitosan-Based Mucoadhesive Vaginal Tablets for Controlled Release of the Anti-HIV Drug Tenofovir. Pharmaceutics 2019, 11, 20. [Google Scholar] [CrossRef]
- Ruiz-Caro, R.; Veiga-Ochoa, M.D. Characterization and Dissolution Study of Chitosan Freeze-Dried Systems for Drug Controlled Release. Molecules 2009, 14, 4370–4386. [Google Scholar] [CrossRef] [Green Version]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.-P.; Martín-Illana, A.; Ruiz-Caro, R.; Bermejo, P.; Abad, M.-J.; Carro, R.; Bedoya, L.-M.; Tamayo, A.; Rubio, J.; Fernández-Ferreiro, A.; et al. Chitosan and Kappa-Carrageenan Vaginal Acyclovir Formulations for Prevention of Genital Herpes. In Vitro and Ex Vivo Evaluation. Mar. Drugs 2015, 13, 5976–5992. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.W.; Flanner, H.H. Mathematical comparison of dissolution profiles. Pharma Tec. 1996, 20, 64–74. [Google Scholar]
- Mamani, P.L.; Ruiz-Caro, R.; Veiga, M.D. Matrix Tablets: The Effect of Hydroxypropyl Methylcellulose/Anhydrous Dibasic Calcium Phosphate Ratio on the Release Rate of a Water-Soluble Drug Through the Gastrointestinal Tract I. In Vitro Tests. AAPS PharmSciTech 2012, 13, 1073–1083. [Google Scholar] [CrossRef] [Green Version]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.-M.; Tamayo, A.; Rubio, J.; Veiga, M.-D.; Harding, D.; Sashiwa, H. Influence of Chitosan Swelling Behaviour on Controlled Release of Tenofovir from Mucoadhesive Vaginal Systems for Prevention of Sexual Transmission of HIV. Mar. Drugs 2017, 15, 50. [Google Scholar] [CrossRef]
- Ochiuz, L.; Grigoras, C.; Popa, M.; Stoleriu, I.; Munteanu, C.; Timofte, D.; Profire, L.; Grigoras, A.G. Alendronate-Loaded Modified Drug Delivery Lipid Particles Intended for Improved Oral and Topical Administration. Molecules 2016, 21, 858. [Google Scholar] [CrossRef]
- Eloy, J.D.O.; Saraiva, J.; De Albuquerque, S.; Marchetti, J.M.; Albuquerque, S. Solid Dispersion of Ursolic Acid in Gelucire 50/13: A Strategy to Enhance Drug Release and Trypanocidal Activity. AAPS PharmSciTech 2012, 13, 1436–1445. [Google Scholar] [CrossRef]
- Patil, S.; Kadam, C.; Pokharkar, V. QbD based approach for optimization of Tenofovir disoproxil fumarate loaded liquid crystal precursor with improved permeability. J. Adv. Res. 2017, 8, 607–616. [Google Scholar] [CrossRef]
- Ramkumaar, G.R.; Srinivasan, S.; Bhoopathy, T.J.; Gunasekaran, S. Vibrational Spectroscopic Studies of Tenofovir Using Density Functional Theory Method. J. Chem. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Svensson, A.; Neves, C.; Cabane, B. Hydration of an amphiphilic excipient, Gelucire® 44/14. Int. J. Pharm. 2004, 281, 107–118. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Database, CID=464205. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/464205 (accessed on 1 July 2017).
- Veiga, M.; Bernad, M.; Escobar, C. Thermal behaviour of drugs from binary and ternary systems. Int. J. Pharm. 1993, 89, 119–124. [Google Scholar] [CrossRef]
- Agrahari, V.; Putty, S.; Mathes, C.; Murowchick, J.B.; Youan, B.-B.C. Evaluation of degradation kinetics and physicochemical stability of tenofovir. Drug Test. Analysis 2014, 7, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Albarahmieh, E.; Qi, S.; Craig, D.Q. Hot melt extruded transdermal films based on amorphous solid dispersions in Eudragit RS PO: The inclusion of hydrophilic additives to develop moisture-activated release systems. Int. J. Pharm. 2016, 514, 270–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Zhang, C.; Agrahari, V.; Murowchick, J.B.; Oyler, N.A.; Youan, B.B.C. Spray drying tenofovir loaded mucoadhesive and pH-sensitive microspheres intended for HIV prevention. Antivir. Res. 2013, 97, 334. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Li, H.; Guo, Z.; Wu, L.; Chen, F.; De Matas, M.; Shao, Q.; Xiao, T.; York, P.; He, Y.; et al. Quantification of Swelling and Erosion in the Controlled Release of a Poorly Water-Soluble Drug Using Synchrotron X-ray Computed Microtomography. AAPS J 2013, 15, 1025–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geeta, M.P.; Madhabhai, M.P. Design and in vitro evaluation of a novel vaginal drug delivery system based on gelucire. Curr Drug Deliv 2009, 6, 159–165. [Google Scholar] [PubMed]
- Stevens, R.E.; Gray, V.; Dorantes, A.; Gold, L.; Pham, L. Scientific and Regulatory Standards for Assessing Product Performance Using the Similarity Factor, f2. AAPS J 2015, 17, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, D. Elaborations on the Higuchi model for drug delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Hixson, A.W.; Crowell, J.H. Dependence of reaction velocity upon surface and agitation: Theoretical considerations. Ind Eng Chem. 1931, 23, 923–931. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef]












| Granules | Tenofovir | Gelucire® 39 | Gelucire® 43 |
|---|---|---|---|
| TFV-1-G43-2 | 30 | 60 | |
| TFV-1-G43-1 | 30 | 30 | |
| TFV-2-G43-1 | 30 | 15 | |
| TFV-1-G41-2 | 30 | 30 | 30 |
| TFV-1-G39-2 | 30 | 60 |
| Batch | HPMC | CH | ACDP | PVP | TFV | G43 | G39 | MgSt |
|---|---|---|---|---|---|---|---|---|
| TFV-1-G43-2 C1 | 145 | 145 | 45 | 27 | 30 | 60 | 3 | |
| TFV-1-G43-2 C2 | 190 | 100 | 45 | 27 | 30 | 60 | 3 | |
| TFV-1-G43-2 C3 | 100 | 190 | 45 | 27 | 30 | 60 | 3 | |
| TFV-1-G43-1 C1 | 145 | 145 | 45 | 27 | 30 | 30 | 3 | |
| TFV-1-G43-1 C2 | 190 | 100 | 45 | 27 | 30 | 30 | 3 | |
| TFV-1-G43-1 C3 | 100 | 190 | 45 | 27 | 30 | 30 | 3 | |
| TFV-2-G43-1 C1 | 145 | 145 | 45 | 27 | 30 | 15 | 3 | |
| TFV-2-G43-1 C2 | 190 | 100 | 45 | 27 | 30 | 15 | 3 | |
| TFV-2-G43-1 C3 | 100 | 190 | 45 | 27 | 30 | 15 | 3 | |
| TFV-1-G41-2 C1 | 145 | 145 | 45 | 27 | 30 | 30 | 30 | 3 |
| TFV-1-G41-2 C2 | 190 | 100 | 45 | 27 | 30 | 30 | 30 | 3 |
| TFV-1-G41-2 C3 | 100 | 190 | 45 | 27 | 30 | 30 | 30 | 3 |
| TFV-1-G39-2 C1 | 145 | 145 | 45 | 27 | 30 | 60 | 3 | |
| TFV-1-G39-2 C2 | 190 | 100 | 45 | 27 | 30 | 60 | 3 | |
| TFV-1-G39-2 C3 | 100 | 190 | 45 | 27 | 30 | 60 | 3 |
| REFERENCE | PROBLEM | f2 | REFERENCE | PROBLEM | f2 | REFERENCE | PROBLEM | f2 |
|---|---|---|---|---|---|---|---|---|
| C1 | T1G(43)2 C1 | 44.6 | C1 | C2 | 84.3 | T1G(43)2 C1 | T1G(43)2 C2 | 83.4 |
| C1 | T1G(43)1 C1 | 64.7 | C1 | C3 | 76.9 | T1G(43)2 C1 | T1G(43)2 C3 | 78.4 |
| C1 | T2G(43)1 C1 | 66.9 | C2 | C3 | 85.6 | T1G(43)2 C2 | T1G(43)2 C3 | 68.1 |
| C1 | T1G(41)2 C1 | 60.4 | T1G(43)2 C1 | T1G(43)1 C1 | 54.5 | T1G(43)1 C1 | T1G(43)1 C2 | 83.5 |
| C1 | T1G(39)2 C1 | 60.5 | T1G(43)2 C1 | T2G(43)1 C1 | 53.7 | T1G(43)1 C1 | T1G(43)1 C3 | 84.9 |
| C2 | T1G(43)2 C2 | 40.9 | T1G(43)2 C1 | T1G(41)2 C1 | 56.3 | T1G(43)1 C2 | T1G(43)1 C3 | 75.1 |
| C2 | T1G(43)1 C2 | 55.8 | T1G(43)2 C1 | T1G(39)2 C1 | 57.2 | T2G(43)1 C1 | T2G(43)1 C2 | 81.3 |
| C2 | T2G(43)1 C2 | 56.7 | T1G(43)2 C2 | T1G(43)1 C2 | 50.0 | T2G(43)1 C1 | T2G(43)1 C3 | 94.5 |
| C2 | T1G(41)2 C2 | 60.5 | T1G(43)2 C2 | T2G(43)1 C2 | 51.0 | T2G(43)1 C2 | T2G(43)1 C3 | 84.8 |
| C2 | T1G(39)2 C2 | 54.7 | T1G(43)2 C2 | T1G(41)2 C2 | 48.4 | T1G(41)2 C1 | T1G(41)2 C2 | 86.2 |
| C3 | T1G(43)2 C3 | 43.3 | T1G(43)2 C2 | T1G(39)2 C2 | 50.0 | T1G(41)2 C1 | T1G(41)2 C3 | 73.7 |
| C3 | T1G(43)1 C3 | 60.5 | T1G(43)2 C3 | T1G(43)1 C3 | 54.6 | T1G(41)2 C2 | T1G(41)2 C3 | 83.7 |
| C3 | T2G(43)1 C3 | 56.5 | T1G(43)2 C3 | T2G(43)1 C3 | 59.1 | T1G(39)2 C1 | T1G(39)2 C2 | 88.1 |
| C3 | T1G(41)2 C3 | 61.9 | T1G(43)2 C3 | T1G(41)2 C3 | 52.4 | T1G(39)2 C1 | T1G(39)2 C3 | 62.7 |
| C3 | T1G(39)2 C3 | 64.1 | T1G(43)2 C3 | T1G(39)2 C3 | 49.3 | T1G(39)2 C2 | T1G(39)2 C3 | 67.4 |
| Batch | Correlation Coefficients (r2) | |||||
|---|---|---|---|---|---|---|
| Zero Order | First Order | Higuchi | Hixson-Crowell | Hopfenberg | Korsmeyer-Peppas | |
| TFV1G(43)2 C1 | 0.9557 | 0.7162 | 0.9975 | 0.9888 | 0.9824 | 0.9915 |
| TFV1G(43)2 C2 | 0.9658 | 0.7139 | 0.9961 | 0.9925 | 0.9877 | 0.9876 |
| TFV1G(43)2 C3 | 0.9567 | 0.7188 | 0.9971 | 0.9922 | 0.9858 | 0.9911 |
| TFV1G(43)1 C1 | 0.9140 | 0.6436 | 0.9885 | 0.9749 | 0.9624 | 0.9795 |
| TFV1G(43)1 C2 | 0.9298 | 0.6610 | 0.9911 | 0.9884 | 0.9771 | 0.9887 |
| TFV1G(43)1 C3 | 0.9110 | 0.6514 | 0.9878 | 0.9835 | 0.9690 | 0.9896 |
| TFV2G(43)1 C1 | 0.9190 | 0.6474 | 0.9920 | 0.9852 | 0.9723 | 0.9801 |
| TFV2G(43)1 C2 | 0.9351 | 0.6671 | 0.9954 | 0.9911 | 0.9813 | 0.9864 |
| TFV2G(43)1 C3 | 0.9306 | 0.6849 | 0.9939 | 0.9938 | 0.9834 | 0.9933 |
| TFV1G(41)2 C1 | 0.9377 | 0.663 | 0.9930 | 0.9958 | 0.9877 | 0.9850 |
| TFV1G(41)2 C2 | 0.9307 | 0.6604 | 0.9912 | 0.9963 | 0.9897 | 0.9889 |
| TFV1G(41)2 C3 | 0.9148 | 0.6139 | 0.9886 | 0.9918 | 0.9775 | 0.9662 |
| TFV1G(39)2 C1 | 0.9384 | 0.6522 | 0.9886 | 0.9948 | 0.9850 | 0.9862 |
| TFV1G(39)2 C2 | 0.9270 | 0.6708 | 0.9854 | 0.9916 | 0.9796 | 0.9941 |
| TFV1G(39)2 C3 | 0.9005 | 0.6437 | 0.9819 | 0.9879 | 0.9705 | 0.9983 |
| Batch | Higuchi | Hixson-Crowell | Hopfenberg | Korsmeyer-Peppas | |
|---|---|---|---|---|---|
| KH | KHC | KHF | KKP | n | |
| TFV1G(43)2 C1 | 0.083 | 0.0040 | 0.0054 | 0.042 | 0.68 |
| TFV1G(43)2 C2 | 0.080 | 0.0039 | 0.0052 | 0.036 | 0.71 |
| TFV1G(43)2 C3 | 0.088 | 0.0045 | 0.0060 | 0.046 | 0.67 |
| TFV1G(43)1 C1 | 0.102 | 0.0059 | 0.0075 | 0.045 | 0.82 |
| TFV1G(43)1 C2 | 0.103 | 0.0060 | 0.0076 | 0.037 | 0.86 |
| TFV1G(43)1 C3 | 0.106 | 0.0065 | 0.0081 | 0.048 | 0.81 |
| TFV2G(43)1 C1 | 0.103 | 0.0061 | 0.0077 | 0.056 | 0.73 |
| TFV2G(43)1 C2 | 0.108 | 0.0058 | 0.0074 | 0.047 | 0.78 |
| TFV2G(43)1 C3 | 0.105 | 0.0065 | 0.0081 | 0.052 | 0.75 |
| TFV1G(41)2 C1 | 0.107 | 0.0067 | 0.0083 | 0.038 | 0.85 |
| TFV1G(41)2 C2 | 0.111 | 0.0076 | 0.0091 | 0.040 | 0.86 |
| TFV1G(41)2 C3 | 0.110 | 0.0073 | 0.0089 | 0.043 | 0.88 |
| TFV1G(39)2 C1 | 0.108 | 0.0066 | 0.0083 | 0.026 | 0.97 |
| TFV1G(39)2 C2 | 0.109 | 0.0067 | 0.0084 | 0.029 | 0.93 |
| TFV1G(39)2 C3 | 0.112 | 0.0075 | 0.0091 | 0.042 | 0.93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Peña, J.; Veiga, M.-D. Tenofovir Hot-Melt Granulation using Gelucire® to Develop Sustained-Release Vaginal Systems for Weekly Protection against Sexual Transmission of HIV. Pharmaceutics 2019, 11, 137. https://doi.org/10.3390/pharmaceutics11030137
Notario-Pérez F, Cazorla-Luna R, Martín-Illana A, Ruiz-Caro R, Peña J, Veiga M-D. Tenofovir Hot-Melt Granulation using Gelucire® to Develop Sustained-Release Vaginal Systems for Weekly Protection against Sexual Transmission of HIV. Pharmaceutics. 2019; 11(3):137. https://doi.org/10.3390/pharmaceutics11030137
Chicago/Turabian StyleNotario-Pérez, Fernando, Raúl Cazorla-Luna, Araceli Martín-Illana, Roberto Ruiz-Caro, Juan Peña, and María-Dolores Veiga. 2019. "Tenofovir Hot-Melt Granulation using Gelucire® to Develop Sustained-Release Vaginal Systems for Weekly Protection against Sexual Transmission of HIV" Pharmaceutics 11, no. 3: 137. https://doi.org/10.3390/pharmaceutics11030137
APA StyleNotario-Pérez, F., Cazorla-Luna, R., Martín-Illana, A., Ruiz-Caro, R., Peña, J., & Veiga, M. -D. (2019). Tenofovir Hot-Melt Granulation using Gelucire® to Develop Sustained-Release Vaginal Systems for Weekly Protection against Sexual Transmission of HIV. Pharmaceutics, 11(3), 137. https://doi.org/10.3390/pharmaceutics11030137