Development of a Transdermal Delivery System for Tenofovir Alafenamide, a Prodrug of Tenofovir with Potent Antiviral Activity Against HIV and HBV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Slide Crystallization Studies
2.2.2. Preparation of Drug in Adhesive Patches of TAF
Formulation of Acrylate-Based Patches
Microscopy of Acrylate-Based Patches
Formulation of TAF Suspension Patches
Visual Observations of the TAF Suspension Patches
Effect of Homogenization on Particle Size of TAF
2.2.3. Coat Weight and TAF Content of the Patches
2.2.4. In Vitro Skin Permeation Studies
Separation of Epidermis
Epidermal Integrity and Thickness Assessment
Selection of the Receptor Solution
In Vitro Skin Permeation Set Up
2.2.5. In Vitro Drug Release Studies
2.2.6. Coat Thickness, Coat Weight, and Drug Content of Optimized Patch
2.2.7. Quantitative Analysis
2.2.8. Physical Characterizations of Optimized Patch
Peel Adhesion
Tack Properties
2.2.9. Evaluation of Skin Irritation Potential of Optimized Patch
2.2.10. Data Analysis
3. Results and Discussion
3.1. Slide Crystallization Studies
3.2. Formulation of TAF Acrylate Patches
Microscopy and Stability Assessment of TAF Acrylate Patches
3.3. Formulation of TAF Suspension Patches
3.3.1. Visual Observation of TAF Suspension Patches
3.3.2. Effect of Homogenization on the Particle Size of TAF
3.4. Coat Weight and Drug Content
3.5. In Vitro Permeation Studies
3.5.1. Epidermal Integrity and Thickness Assessment
3.5.2. Selection of Receptor Solution
3.5.3. Determination of Permeation Flux of TAF Transdermal Patches
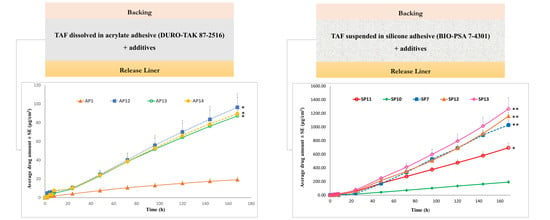
Permeation of TAF from Acrylate-Based Clear Patches: Effect of Drug Concentration
Permeation of TAF from Acrylate-Based Clear Patches: Effect of Coat Weight and Chemical Penetration Enhancers
Permeation of TAF from Suspension-Type Patches: PIB vs Silicone-Based
Permeation of TAF from Silicone-Based Suspension-Type Patches: Effect of Homogenization
Permeation of TAF from Silicone-Based Suspension-Type Patches: Effect of Drug Concentration
Permeation of TAF from Acrylate-Based Clear vs Silicone-Based Suspension-Type Patches
3.6. In Vitro Drug Release Studies
3.7. Coat Thickness, Coat Weight, and Drug Content of Optimized Patch
3.8. Physical Characterization of the Optimized Patch
3.8.1. Peel Adhesion
3.8.2. Tack Properties
3.9. Evaluation of Skin Irritation Potential of Optimized Patch
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| EPI-200-SIT | EpiDermTM skin irritation test |
| ELISA | Enzyme linked immunosorbent assay |
| EVAC | Ethylene vinyl acetate copolymer |
| HPLC | High performance liquid chromatography |
| PBS | Phosphate buffered saline |
| PDMS | Polydimethyl-siloxane |
| PEG | Polyethylene glycol |
| PET | poly-ethylene terephthalate |
| PIB | Polyisobutylene |
| PSA | Pressure sensitive adhesives |
| RhE | Reconstructed human epidermis |
| RT | Room temperature |
| STRs | Single tablet regimens |
| TFV | Tenofovir |
| TAF | Tenofovir alafenamide |
| THF | Tetrahydrofuran |
References
- De Clercq, E. Role of tenofovir alafenamide (TAF) in the treatment and prophylaxis of HIV and HBV infections. Biochem. Pharmacol. 2018, 153, 2–11. [Google Scholar] [CrossRef]
- DeJesus, E.; Haas, B.; Segal-Maurer, S.; Ramgopal, M.N.; Mills, A.; Margot, N.; Liu, Y.-P.; Makadzange, T.; McCallister, S. Superior Efficacy and Improved Renal and Bone Safety After Switching from a Tenofovir Disoproxil Fumarate- to a Tenofovir Alafenamide-Based Regimen Through 96 Weeks of Treatment. AIDS Res. Hum. Retrovir. 2018, 34, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kahn, P. AIDS Vaccine Handbook: Global Perspectives. Available online: http://www.avac.org/ (accessed on 2 November 2019).
- Nelson, K.; Varadarajan, P.; Rajappan, M.; Chikkanna, N. Advances in Drug Delivery of Anti-HIV Drugs—An Overview. Am. J. PharmTech Res. 2012, 2, 231–244. [Google Scholar]
- Vedha Hari, B.N.; Devendharan, K.; Narayanan, N. Approaches of novel drug delivery systems for Anti-HIV agents. Int. J. Drug Dev. Res. 2013, 5, 16–24. [Google Scholar]
- Dou, H.; Destache, C.J.; Morehead, J.R.; Mosley, R.L.; Boska, M.D.; Kingsley, J.; Gorantla, S.; Poluektova, L.; Nelson, J.A.; Chaubal, M.; et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood 2006, 108, 2827–2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunawardana, M.; Remedios-Chan, M.; Miller, C.S.; Fanter, R.; Yang, F.; Marzinke, M.A.; Hendrix, C.W.; Beliveau, M.; Moss, J.A.; Smith, T.J.; et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob. Agents Chemother. 2015, 59, 3913–3919. [Google Scholar] [CrossRef]
- Ball, C.; Krogstad, E.; Chaowanachan, T.; Woodrow, K.A. Drug-Eluting Fibers for HIV-1 Inhibition and Contraception. PLoS ONE 2012, 7, e49792. [Google Scholar] [CrossRef] [PubMed]
- Rohan, L.C.; Devlin, B.; Yang, H. Microbicide Dosage Forms. In Microbicides for Prevention of HIV Infection; Springer: Berlin/Heidelberg, Germany, 2013; pp. 27–54. ISBN 978-3-662-44596-9. [Google Scholar]
- Nelson, A.G.; Zhang, X.; Ganapathi, U.; Szekely, Z.; Flexner, C.W.; Owen, A.; Sinko, P.J. Drug delivery strategies and systems for HIV/AIDS pre-exposure prophylaxis and treatment. J. Control. Release 2015, 219, 669–680. [Google Scholar] [CrossRef]
- Karim, Q.A.; Karim, S.S.A.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.M.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef]
- Puri, A.; Sivaraman, A.; Zhang, W.; Clark, M.R.; Banga, A.K. Expanding the domain of drug delivery for HIV prevention: Exploration of the transdermal route. Crit. Rev. Ther. Drug Carrier Syst. 2017, 34, 551–587. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.S.A.; Kashuba, A.D.; Werner, L.; Karim, Q.A. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: Implications for HIV prevention in women. Lancet 2011, 378, 279–281. [Google Scholar] [CrossRef]
- Banga, A.K. Transdermal and Intradermal Delivery of Therapeutic Agents: Application of Physical Technologies; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781439805107. [Google Scholar]
- Ham, A.S.; Buckheit, R.W. Current and emerging formulation strategies for the effective transdermal delivery of HIV inhibitors. Ther. Deliv. 2015, 6, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Alexander, A.; Dwivedi, S.; Giri, T.K.; Saraf, S.; Saraf, S.; Tripathi, D.K. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J. Control. Release 2012, 164, 26–40. [Google Scholar] [CrossRef]
- Pastore, M.; Kalia, Y.; Hortsmann, M.; Roberts, M. Transdermal patches: history, development and pharmacology. Br. J. Phamacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E. Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem. Pharmacol. 2016, 119, 1–7. [Google Scholar] [CrossRef]
- Murphy, R.A.; Valentovic, M.A. Factors Contributing to the Antiviral Effectiveness of Tenofovir. J. Pharmacol. Exp. Ther. 2017, 363, 156–163. [Google Scholar] [CrossRef]
- Babusis, D.; Phan, T.K.; Lee, W.A.; Watkins, W.J.; Ray, A.S. Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340. Mol. Pharm. 2013, 10, 459–466. [Google Scholar] [CrossRef]
- Foreman, P.; Hansen, A.; Silverberg, E.; Manegold, T.; Yang, H.; Li, J.; Jacobson, S. Predicting Drug Solubility in Transdermal Adhesives. American Association of Pharmaceutical Scientists. In Proceedings of the 37th AAPS Annual Pharmaceutical Technologies Conference, New York, NY, USA, 13–18 January 2002. [Google Scholar]
- Sachdeva, V.; Bai, Y.; Kydonieus, A.; Banga, A.K. Formulation and optimization of desogestrel transdermal contraceptive patch using crystallization studies. Int. J. Pharm. 2013, 441, 9–18. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyay, P.; Ghosh, A.; Bhattacharya, S.; Kundu, A.; Veer, V. Accelerated stability testing of a transdermal patch composed of eserine and pralidoxime chloride for prophylaxis against (±)-anatoxin A poisoning. J. Food Drug Anal. 2014, 22, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Kassis, V.; Søndergaard, J. Heat-separation of normal human skin for epidermal and dermal prostaglandin analysis. Arch. Dermatol. Res. 1982, 273, 301–306. [Google Scholar] [CrossRef]
- Puri, A.; Murnane, K.S.; Blough, B.E.; Banga, A.K. Effects of chemical and physical enhancement techniques on transdermal delivery of 3-fluoroamphetamine hydrochloride. Int. J. Pharm. 2017, 528, 452–462. [Google Scholar] [CrossRef]
- Lakhani, P.; Bahl, R.; Bafna, P. Transdermal Patches: Physiochemical and in-vitro Evaluation Methods. Int. J. Pharm. Sci. Res. 2015, 6, 1826–1836. [Google Scholar]
- Cai, B.; Söderkvist, K.; Engqvist, H.; Bredenberg, S. A new drug release method in early development of transdermal drug delivery systems. Pain Res. Treat. 2012, 2012, 953140. [Google Scholar] [CrossRef]
- Wokovich, A.M.; Prodduturi, S.; Doub, W.H.; Hussain, A.S.; Buhse, L.F. Transdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy and quality attribute. Eur. J. Pharm. Biopharm. 2006, 64, 1–8. [Google Scholar]
- Lu, Z.; Fassihi, R. Influence of colloidal silicon dioxide on gel strength, robustness, and adhesive properties of diclofenac gel formulation for topical application. AAPS PharmSciTech 2015, 16, 636–644. [Google Scholar] [CrossRef]
- Kandárová, H.; Hayden, P.; Klausner, M.; Kubilus, J.; Sheasgreen, J. An in vitro skin irritation test (SIT) using the EpiDerm reconstructed human epidermal (RHE) model. J. Vis. Exp. JoVE 2009, 29, e1336. [Google Scholar]
- Banerjee, S.; Chattopadhyay, P.; Ghosh, A.; Datta, P.; Veer, V. Aspect of adhesives in transdermal drug delivery systems. Int. J. Adhes. Adhes. 2014, 50, 70–84. [Google Scholar]
- Pocius, A. Adhesives. In Kirk-Othmer Encyclopedia of Chemical Technology; Howe-Grants, M., Ed.; Wiley-Interscience: New York, NY, USA, 1991; pp. 445–466. [Google Scholar]
- Lobo, S.; Sachdeva, S.; Goswami, T. Role of pressure-sensitive adhesives in transdermal drug delivery systems. Ther. Deliv. 2016, 7, 33–48. [Google Scholar] [CrossRef]
- Weng, W.; Quan, P.; Liu, C.; Zhao, H.; Fang, L. Design of a Drug-in-Adhesive Transdermal Patch for Risperidone: Effect of Drug-Additive Interactions on the Crystallization Inhibition and In Vitro/In Vivo Correlation Study. J. Pharm. Sci. 2016, 10, 3153–3161. [Google Scholar] [CrossRef]
- Jain, P.; Banga, A.K. Induction and inhibition of crystallization in drug-in-adhesive-type transdermal patches. Pharm. Res. 2013, 30, 562–571. [Google Scholar] [CrossRef]
- Saini, S.; Shikha, B.; Chauhan, S.S. Recent development in penetration enhancers and techniques in transdermal drug delivery system. J. Adv. Pharm. Educ. Res. 2014, 4, 31–40. [Google Scholar]
- Pathan, I.B.; Setty, C.M. Chemical penetration enhancers for transdermal drug delivery systems. Trop. J. Pharm. Res. 2009, 8, 173–179. [Google Scholar] [CrossRef]
- Mitragotri, S. Synergistic effect of enhancers for transdermal drug delivery. Pharm. Res. 2000, 17, 1354–1359. [Google Scholar] [CrossRef]
- Santoyo, S.; Arellano, A.; Ygartua, P.; Martin, C. Penetration enhancer effects on the in vitro percutaneous absorption of piroxicam through rat skin. Int. J. Pharm. 1995, 117, 219–224. [Google Scholar] [CrossRef]
- Andega, S.; Kanikkannan, N.; Singh, M. Comparison of the effect of fatty alcohols on the permeation of melatonin between porcine and human skin. J. Control. Release 2001, 77, 17–25. [Google Scholar] [CrossRef]
- Patel, K.N.; Patel, H.K.; Patel, V.A. Formulation and characterization of drug in adhesive transdermal patches of diclofenac acid. Int. J. Pharm. Pharm. Sci. 2012, 4, 296–299. [Google Scholar]
- Moghimipour, E.; Rezaee, S.; Omidi, A. The effect of formulation factors on the release of oxybutynin hydrochloride from transdermal polymeric patches. J. Appl. Pharm. Sci. 2011, 1, 73–76. [Google Scholar]
- Nicoli, S.; Penna, E.; Padula, C.; Colombo, P.; Santi, P. New transdermal bioadhesive film containing oxybutynin: In vitro permeation across rabbit ear skin. Int. J. Pharm. 2006, 325, 2–7. [Google Scholar] [CrossRef]
- Nicolazzo, J.A.; Morgan, T.M.; Reed, B.L.; Finnin, B.C. Synergistic enhancement of testosterone transdermal delivery. J. Control. Release 2005, 103, 577–585. [Google Scholar] [CrossRef]
- Fraser, I.S.; Weisberg, E.; Kumar, N.; Kumar, S.; Humberstone, A.J.; McCrossin, L.; Shaw, D.; Tsong, Y.Y.; Sitruk-Ware, R. An initial pharmacokinetic study with a Metered Dose Transdermal System® for delivery of the progestogen Nestorone® as a possible future contraceptive. Contraception 2007, 76, 432–438. [Google Scholar] [CrossRef]
- European Meedicines Agency. Guideline on quality of transdermal patches Guideline on quality of transdermal patches Table of contents. Eur. Med. Agency 2014, 44, 1–28. [Google Scholar]
- Sekikawa, H.; Nakano, M.; Arita, T. Inhibitory effect of polyvinylpyrrolidone on the crystallization of drugs. Chem. Pharm. Bull. 1978, 26, 118–126. [Google Scholar] [CrossRef]
- Gong, K.; Viboonkiat, R.; Rehman, I.U.; Buckton, G.; Darr, J.A. Formation and characterization of porous indomethacin-PVP coprecipitates prepared using solvent-free supercritical fluid processing. J. Pharm. Sci. 2005, 94, 2583–2590. [Google Scholar] [CrossRef]
- Ohm, A. Interaction of Bay t 3839 coprecipitates with insoluble excipients. Eur. J. Pharm. Biopharm. 2000, 49, 183–189. [Google Scholar] [CrossRef]
- Ma, X.; Taw, J.; Chiang, C.M. Control of drug crystallization in transdermal matrix system. Int. J. Pharm. 1996, 142, 115–119. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 2362–2373. [Google Scholar] [CrossRef] [Green Version]
- Dhankhar, P. Homogenization Fundamentals. IOSR J. Eng. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Chu, K.R.; Lee, E.; Jeong, S.H.; Park, E.-S. Effect of particle size on the dissolution behaviors of poorly water-soluble drugs. Arch. Pharm. Res. 2012, 35, 1187–1195. [Google Scholar] [CrossRef]
- Anarjan, N.; Jafarizadeh-Malmiri, H.; Nehdi, I.A.; Sbihi, H.M.; Al-Resayes, S.I.; Tan, C.P. Effects of homogenization process parameters on physicochemical properties of astaxanthin nanodispersions prepared using a solvent-diffusion technique. Int. J. Nanomed. 2015, 10, 1109–1118. [Google Scholar] [Green Version]
- Sharma, N.; Madan, P.; Lin, S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, J.N. Electrical resistance and tritiated water permeability as indicators of barrier integrity of in vitro human skin. Toxicol. Vitr. 1997, 11, 241–249. [Google Scholar] [CrossRef]
- Golla, V.M.; Kurmi, M.; Shaik, K.; Singh, S. Stability behaviour of antiretroviral drugs and their combinations. 4: Characterization of degradation products of tenofovir alafenamide fumarate and comparison of its degradation and stability behaviour with tenofovir disoproxil fumarate. J. Pharm. Biomed. Anal. 2016, 131, 146–155. [Google Scholar] [CrossRef]
- Larrucea, E.; Arellano, A.; Santoyo, S.; Ygartua, P. Combined effect of oleic acid and propylene glycol on the percutaneous penetration of tenoxicam and its retention in the skin. Eur. J. Pharm. Biopharm. 2001, 2, 113–119. [Google Scholar] [CrossRef]
- Benson, H.A.E. Transdermal drug delivery: Penetration enhancement techniques. Curr. Drug Deliv. 2005, 2, 23–33. [Google Scholar] [CrossRef]
- Chatterjee, D.J.; Li, W.Y.; Koda, R.T. Effect of vehicles and penetration enhancers on the in vitro and in vivo percutaneous absorption of methotrexate and edatrexate through hairless mouse skin. Pharm. Res. 1997, 14, 1058–1065. [Google Scholar] [CrossRef]
- Quan, D.; Deshpanday, N.; Venkateshwaran, S.; Ebert, C. Triacetin as a Penetration Enhancer for Transdermal Delivery of a Basic Drug. U.S. Patent No 5,834,010, 10 November 1998. [Google Scholar]
- Cilurzo, F.; Gennari, C.G.M.; Minghetti, P. Adhesive properties: A critical issue in transdermal patch development. Expert Opin. Drug Deliv. 2012, 9, 33–45. [Google Scholar] [CrossRef]
- Miller, K.; Govil, S.; Bhatia, K. Fentanyl Suspension-Based Silicone Adhesive Formulations and Devices for Transdermal Delivery of Fentanyl. U.S. Patent 7556823B2, 28 October 2008. [Google Scholar]










| Properties | Patch Formulation Codes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AP1 | AP2 | AP3 | AP4 | AP5 | AP6 | AP7 | AP8 | AP9 | AP10 | AP11 | AP12 | AP13 | AP14 | AP15 | |
| Coat weight (gsm) | 200 | 200 | 200 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 400 | 400 | 400 | 400 |
| Composition | Components (% Dry Weight, w/w) | ||||||||||||||
| TAF | 2 | 3 | 4 | 5 | 10 | 15 | 10 | 10 | 10 | 7.5 | 7.5 | 2 | 2 | 2 | 2 |
| OA | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | - |
| DURO-TAK 87-2516 | 93 | 92 | 91 | 87.86 | 80.71 | 73.57 | 83.89 | 83.89 | 83.89 | 77.50 | 79.29 | 83 | 83 | 79.5 | 83 |
| PVP 360 | - | - | - | 2.14 | 4.29 | 6.43 | - | - | - | 5 | - | - | - | - | - |
| Kollidon® VA64 | - | - | - | - | - | - | 1.11 | - | - | - | - | - | - | - | - |
| Kollidon® 90 F | - | - | - | - | - | - | - | 1.11 | - | - | - | - | - | - | - |
| Kollidon® 30 LP | - | - | - | - | - | - | - | - | 1.11 | - | 3.21 | - | - | - | - |
| Oleyl alcohol | - | - | - | - | - | - | - | - | - | - | - | 5 | - | - | 10 |
| PG | - | - | - | - | - | - | - | - | - | 5 | 5 | 5 | 5 | 5 | 5 |
| Triacetin | - | - | - | - | - | - | - | - | - | - | - | - | 5 | - | - |
| Octisalate | - | - | - | - | - | - | - | - | - | - | - | - | - | 8.5 | - |
| Properties | Patch Formulation Codes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SP1 | SP2 | SP3 | SP4 | SP5 | SP6 | SP7 | SP8 | SP9 | SP10 | SP11 | SP12 | SP13 | SP14 | |
| Composition of matrix | Components (% Dry Weight, w/w) | |||||||||||||
| TAF | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 5 | 10 | 20 | 25 | 15 |
| OA | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Oleyl alcohol | - | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | - |
| Mineral oil | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| BIO-PSA 7-4301 | 66 | 56 | 56 | 56 | 56 | 56 | 56 | 56 | 56 | 66 | 61 | 51 | 46 | - |
| DURO-TAK 87-6908 | - | - | - | - | - | - | - | - | - | - | - | - | - | 66 |
| Coat weight (gsm) | ~100 | ~250 | ~250 | ~250 | ~250 | ~250 | ~300 | ~300 | ~300 | ~350 | ~350 | ~200 | ~200 | ~50 |
| Release liner | Fluoro-polymer coated side of ScotchpakTM 1022 | Uncoated side of ScotchpakTM 1022 | Fluoro-polymer coated side of ScotchpakTM 1022 | |||||||||||
| Backing membrane | ScotchpakTM 9733 (polyester) | ScotchpakTM 9733 (polyester) | CoTran™ 9702, 9706, 9728 (EVAC) | CoTran™ 9720 (poly-ethylene) | CoTran™ 9718 (poly-ethylene) | CoTran™ 9718 (poly-ethylene) | Silicone coated PET film (48101) | Silicone coated PET film (44916) | Silicone coated PET film (40987) | Silicone coated PET film (48101) | ScotchpakTM 9733 (polyester) | |||
| Homogenization speed (rpm) and time (min) before addition of adhesive | 5000; 3 | 30,000; 20 | 32,000; 30 | 32,000; 5 | 5000; 3 | |||||||||
| Homogenization speed (rpm) and time (min) after addition of adhesive | 5000; 1 | 30,000; 1 | 32,000; 1 | 15,000; 1 | 5000; 1 | |||||||||
| Drying conditions | 78 °C for 10 min | Air drying for 5 min. followed by drying at 78 °C for 15 min | Air drying, 15 min | 78 °C for 10 min | ||||||||||
| Patch Formulation Code | COAT WEIGHT (mg/cm2), mean ± SD | DRUG CONTENT (mg/0.64 cm2), mean ± SD | ||
|---|---|---|---|---|
| Targeted | Experimental | Theoretical | Experimental | |
| AP1 | 20 | 18.20 ± 1.88 | 0.256 | 0.25 ± 0.02 |
| AP5 | 10 | 7.10 ± 0.14 | 0.640 | 0.53 ± 0.05 |
| AP12 | 40 | 37.37 ± 6.94 | 0.512 | 0.478 ± 0.09 |
| AP13 | 40 | 40.80 ± 1.47 | 0.512 | 0.522 ± 0.02 |
| AP14 | 40 | 44.17 ± 4.96 | 0.512 | 0.565 ± 0.06 |
| AP15 | 40 | 40.43 ± 2.35 | 0.512 | 0.643 ± 0.106 |
| SP1 | 10 | 12.30 ± 3.15 | 0.96 | 1.35 ± 0.28 |
| SP5 | 25 | 24.23 ± 2.88 | 2.40 | 2.52 ± 0.19 |
| SP6 | 25 | 20.99 ± 5.80 | 2.40 | 2.17 ± 0.55 |
| SP7 | 30 | 29.60 ± 1.94 | 2.88 | 2.94 ± 0.65 |
| SP10 | 35 | 34.15 ± 3.75 | 1.12 | 1.11 ± 0.06 |
| SP11 | 35 | 36.05 ± 1.29 | 2.24 | 2.53 ± 0.11 |
| SP12 | 20 | 16.43 ± 0.39 | 2.56 | 2.31 ± 0.16 |
| SP13 | 20 | 18.29 ± 1.82 | 3.20 | 3.02 ± 0.14 |
| SP14 | 5 | 4.54 ± 0.55 | 0.48 | 0.80 ± 0.06 |
| Patch Codes | Temperature | ||
|---|---|---|---|
| −20 °C | RT | 40 °C | |
| AP1 | NO | NO | NO |
| AP2 | NO | Crystals after three weeks | Crystals after three weeks |
| AP3 | NO | Crystals after two weeks | Crystals after two weeks |
| AP4 | NO | Crystals after three weeks | Crystals after three weeks |
| AP5 | NO | Crystals after two weeks | Crystals after two weeks |
| AP6 | Crystals after 2 months | Crystals after 9 days | Crystals after one week |
| AP7 | Not observed | Crystals in first week | Crystals in first week |
| AP8 | Not observed | Crystals in first week | Crystals in first week |
| AP9 | Not observed | Smaller crystals than AP7 and AP8 after 12 days | Crystals in first week (2–3 crystals) |
| AP10 | NO | Crystals after three weeks | Crystals after two weeks |
| AP11 | NO | Crystals after three weeks | Crystals after two weeks |
| AP12 | NO | NO | NO |
| AP13 | NO | NO | NO |
| AP14 | NO | NO | NO |
| AP15 | NO | NO | NO |
| Formulation Code | Properties | ||||
|---|---|---|---|---|---|
| Phase Separation | Contraction/Shrinkage of Films | Residue on Release Liner after Peeling | Ease of Peeling Patches off the Skin | Residue on the Glove after Patch Removal | |
| SP1 | No | No | No | Yes | No |
| SP2 | No | No | No | Yes | Yes |
| SP4 | No | No | No | Yes | Yes |
| SP5 | No | Yes (after a month) | No | Yes | Not when applied afresh, but if applied after storing for few days |
| SP6 | No | Yes (after a month) | No | Yes | Not when applied afresh, but if applied after storing for few days |
| SP7 | No | No | No | Yes | No |
| SP8 | No | No | No | No | No |
| SP9 | No | No | No | No | No |
| SP10 | No | No | No | Yes | No |
| SP11 | No | No | No | Yes | No |
| SP12 | No | No | No | Yes | No |
| SP13 | No | No | No | Yes | No |
| SP14 | No | No | No | Yes | No |
| TAF Concentration (µg/mL) | % Degradation of TAF in 24 h at 37 °C | |||
|---|---|---|---|---|
| PBS, pH 7.4 | Phosphate Buffer, pH 6.0 | PEG 400: PBS, pH 7.4 (1:1) | PEG 400 | |
| 0.5 | 22.28 | 3.65 | 41.85 | Not detected due to low LOD |
| 5 | 28.29 | 1.61 | 32.40 | 0.00 |
| 50 | 30.54 | 2.27 | 33.84 | 0.85 |
| Parameters | Week 1 (RT) | After 1.5 months (RT) | After 1.5 months (40 °C) | After 3 months (RT) | After 3 months (40 °C) |
|---|---|---|---|---|---|
| % TAF released after 7 days ± SE | 64.27 ± 5.47 | 69.71 ± 5.63 | 66.82 ± 12.83 | 97.43 ± 19.03 | 88.86 ± 7.88 |
| Coat weight (mg/cm2) ± SD | 29.60 ± 1.94 | 30.38 ± 0.95 | 32.73 ± 3.06 | 29.39 ± 4.57 | 33.55 ± 3.14 |
| Coat thickness (µm) ± SD | 182.66 ± 1.15 | 186.67 ± 3.06 | 188.00 ± 3.46 | 186.00 ± 4.00 | 189.33 ± 3.06 |
| Drug content (mg/cm2) ± SD | 4.60 ± 1.01 | 4.95 ± 0.31 | 4.92 ± 0.39 | 5.25 ± 0.32 | 5.36 ± 0.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puri, A.; Bhattaccharjee, S.A.; Zhang, W.; Clark, M.; Singh, O.N.; Doncel, G.F.; Banga, A.K. Development of a Transdermal Delivery System for Tenofovir Alafenamide, a Prodrug of Tenofovir with Potent Antiviral Activity Against HIV and HBV. Pharmaceutics 2019, 11, 173. https://doi.org/10.3390/pharmaceutics11040173
Puri A, Bhattaccharjee SA, Zhang W, Clark M, Singh ON, Doncel GF, Banga AK. Development of a Transdermal Delivery System for Tenofovir Alafenamide, a Prodrug of Tenofovir with Potent Antiviral Activity Against HIV and HBV. Pharmaceutics. 2019; 11(4):173. https://doi.org/10.3390/pharmaceutics11040173
Chicago/Turabian StylePuri, Ashana, Sonalika A. Bhattaccharjee, Wei Zhang, Meredith Clark, Onkar N. Singh, Gustavo F. Doncel, and Ajay K. Banga. 2019. "Development of a Transdermal Delivery System for Tenofovir Alafenamide, a Prodrug of Tenofovir with Potent Antiviral Activity Against HIV and HBV" Pharmaceutics 11, no. 4: 173. https://doi.org/10.3390/pharmaceutics11040173
APA StylePuri, A., Bhattaccharjee, S. A., Zhang, W., Clark, M., Singh, O. N., Doncel, G. F., & Banga, A. K. (2019). Development of a Transdermal Delivery System for Tenofovir Alafenamide, a Prodrug of Tenofovir with Potent Antiviral Activity Against HIV and HBV. Pharmaceutics, 11(4), 173. https://doi.org/10.3390/pharmaceutics11040173