Advantages and Limitations of Integrated Flagellin Adjuvants for HIV-Based Nanoparticle B-Cell Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice, Ethical Statement
2.2. Cell Lines, Plasmids
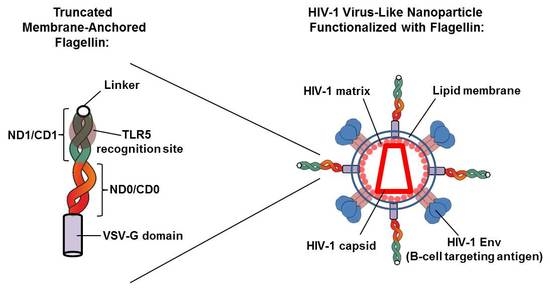
2.3. Construction of an Expression Plasmid Encoding Membrane-Anchored KFΔ (pKFΔ-TM)
2.4. VLP Production and Characterization
2.5. Isolation and Purification of Splenic Cells, In Vitro Culture Experiments
2.6. Immunization Experiments, Collection of Blood Samples
2.7. Analyses of Humoral Immune Responses
2.8. Characterization of Cellular Immune Responses
2.9. Statistical Analysis
3. Results and Discussion
3.1. Generation of a Membrane-Bound Form of Truncated KF for HIV-VLP Functionalization
3.2. Activation of Antigen-Presenting Cells in Vitro
3.3. Modulation of Env-Specific Antibody and CD4+ T-Cell Responses In Vivo
3.4. Adjuvantive Effect on HEL-Specific Antibody Responses In Vivo
3.5. Immunogenicity Balances between the Target Antigens and KFΔ
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Zhao, C.; Ao, Z.; Yao, X.; Zhao, C.; Ao, Z.; Yao, X. Current Advances in Virus-Like Particles as a Vaccination Approach against HIV Infection. Vaccines 2016, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Temchura, V.; Überla, K. Intrastructural help: Improving the HIV-1 envelope antibody response induced by virus-like particle vaccines. Curr. Opin. HIV AIDS 2017, 12, 272–277. [Google Scholar] [CrossRef]
- Asbach, B.; Wagner, R. Particle-based delivery of the HIV envelope protein. Curr. Opin. HIV AIDS 2017, 12, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Westendorf, A.M.; Buer, J.; Überla, K.; Epple, M. The potential of nanoparticles for the immunization against viral infections. J. Mater. Chem. B 2015, 3, 4767–4779. [Google Scholar] [CrossRef] [Green Version]
- Gardt, O.; Grewe, B.; Tippler, B.G.; Überla, K.; Temchura, V.V. HIV-derived lentiviral particles promote T-cell independent activation and differentiation of naïve cognate conventional B2-cells in vitro. Vaccine 2013, 31, 5088–5098. [Google Scholar] [CrossRef] [PubMed]
- Temchura, V.; Kalinin, S.; Nabi, G.; Tippler, B.; Niezold, T.; Überla, K. Divergence of primary cognate B- and T-cell proliferative responses to subcutaneous and intravenous immunization with virus-like particles. Viruses 2014, 6, 3334–3347. [Google Scholar] [CrossRef] [PubMed]
- Nabi, G.; Genannt Bonsmann, M.S.; Tenbusch, M.; Gardt, O.; Barouch, D.H.; Temchura, V.; Überla, K. GagPol-specific CD4+ T-cells increase the antibody response to Env by intrastructural help. Retrovirology 2013, 10, 117. [Google Scholar] [CrossRef]
- Kolenbrander, A.; Grewe, B.; Nemazee, D.; Überla, K.; Temchura, V. Generation of T follicular helper cells in vitro: Requirement for B-cell receptor cross-linking and cognate B- and T-cell interaction. Immunology 2018, 153, 214–224. [Google Scholar] [CrossRef]
- Genannt Bonsmann, M.S.; Niezold, T.; Temchura, V.; Pissani, F.; Ehrhardt, K.; Brown, E.P.; Osei-Owusu, N.Y.; Hannaman, D.; Hengel, H.; Ackerman, M.E.; et al. Enhancing the Quality of Antibodies to HIV-1 Envelope by GagPol-Specific Th Cells. J. Immunol. 2015, 195, 4861–4872. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, H.; Nabi, G.; McKinstry, W.J.; Khoo, K.K.; Mak, J.; Salazar, A.M.; Tenbusch, M.; Temchura, V.; Uberla, K. Intrastructural help: Harnessing T helper cells induced by licensed vaccines for improvement of HIV env antibody responses to virus-like particle vaccines. J. Virol. 2018. [Google Scholar] [CrossRef]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll- like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef]
- Mizel, S.B.; Bates, J.T. Flagellin as an adjuvant: Cellular mechanisms and potential. J. Immunol. 2010. [Google Scholar] [CrossRef]
- Zilker, C.; Kozlova, D.; Sokolova, V.; Yan, H.; Epple, M.; Überla, K.; Temchura, V. Nanoparticle-based B-cell targeting vaccines: Tailoring of humoral immune responses by functionalization with different TLR-ligands. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 173–182. [Google Scholar] [CrossRef]
- Liaudet, L.; Murthy, K.G.K.; Mabley, J.G.; Pacher, P.; Soriano, F.G.; Salzman, A.L.; Szabó, C. Comparison of Inflammation, Organ Damage, and Oxidant Stress Induced by Salmonella enterica Serovar Muenchen Flagellin and Serovar Enteritidis Lipopolysaccharide. Infect. Immun. 2002, 70, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Honko, A.N.; Mizel, S.B. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect. Immun. 2004, 72, 6676–6679. [Google Scholar] [CrossRef]
- Yang, J.; Zhong, M.; Zhang, Y.; Zhang, E.; Sun, Y.; Cao, Y.; Li, Y.; Zhou, D.; He, B.; Chen, Y.; et al. Antigen replacement of domains D2 and D3 in flagellin promotes mucosal IgA production and attenuates flagellin-induced inflammatory response after intranasal immunization. Hum. Vaccines Immunother. 2013, 9, 1084–1092. [Google Scholar] [CrossRef] [Green Version]
- Wagner, R.; Graf, M.; Bieler, K.; Wolf, H.; Grunwald, T.; Foley, P.; Überla, K. Rev-Independent Expression of Synthetic gag-pol Genes of Human Immunodeficiency Virus Type 1 and Simian Immunodeficiency Virus: Implications for the Safety of Lentiviral Vectors. Hum. Gene Ther. 2000, 11, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, W.; Yang, J.Y.; Zhou, D.H.; Chen, Y.Q.; Zhang, Y.; Yang, Y.; He, B.X.; Zhong, M.H.; Li, Y.M.; et al. Flagellin-PAc Fusion Protein is a High-efficacy Anti-caries Mucosal Vaccine. J. Dent. Res. 2012, 91, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Grewe, B.; Hoffmann, B.; Ohs, I.; Blissenbach, M.; Brandt, S.; Tippler, B.; Grunwald, T.; Uberla, K. Cytoplasmic utilization of human immunodeficiency virus type 1 genomic RNA is not dependent on a nuclear interaction with gag. J. Virol. 2012, 86, 2990–3002. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.D.; Andersen-Nissen, E.; Hayashi, F.; Strobe, K.; Bergman, M.A.; Barrett, S.L.R.; Cookson, B.T.; Aderem, A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 2003, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Swartz, J.R. Functional properties of flagellin as a stimulator of innate immunity. Sci. Rep. 2016, 6, 18379. [Google Scholar] [CrossRef] [Green Version]
- Gururajan, M.; Jacob, J.; Pulendran, B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS ONE 2007, 2, e863. [Google Scholar] [CrossRef]
- Coutelier, J.P.; van der Logt, J.T.; Heessen, F.W.; Warnier, G.; Van Snick, J. IgG2a restriction of murine antibodies elicited by viral infections. J. Exp. Med. 1987, 165, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010. [Google Scholar] [CrossRef]
- McSorley, S.J.; Ehst, B.D.; Yu, Y.; Gewirtz, A.T. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J. Immunol. 2002, 169, 3914–3919. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.T.; Uematsu, S.; Akira, S.; Mizel, S.B. Direct stimulation of tlr5+/+ CD11c+ cells is necessary for the adjuvant activity of flagellin. J. Immunol. 2009, 182, 7539–7547. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Katona, I.M.; Mosmann, T.R.; Coffman, R.L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J. Immunol. 1988, 140, 1022–1027. [Google Scholar]
- Vassilieva, E.V.; Wang, B.-Z.; Vzorov, A.N.; Wang, L.; Wang, Y.; Bozja, J.; Xu, R.; Compans, R.W. Enhanced Mucosal Immune Responses to HIV Virus-Like Particles Containing a Membrane-Anchored Adjuvant. mBio 2011, 2, e00328-10. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.W.; Kipp, D.E.; Melchers, I.; Frelinger, J.A.; Sercarz, E.E. Multiple H-2 and non-H-2 genes controlling the antilysozyme response: Alternative gene constellations can lead to responsiveness. Eur. J. Immunol. 1980, 10, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.C.; Kim, E.H.; Luo, W.; Pulendran, B. B Cell Competition for Restricted T Cell Help Suppresses Rare-Epitope Responses. Cell Rep. 2018, 25, 321–327.e3. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, S.; Isobe, M.; Kurosawa, N. Guinea pig immunoglobulin VH and VL naïve repertoire analysis. PLoS ONE 2018, 13, e0208977. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Crooks, E.T.; Osawa, K.; Robinson, J.E.; Barnes, M.; Apetrei, C.; Binley, J.M. Multi-parameter exploration of HIV-1 virus-like particles as neutralizing antibody immunogens in guinea pigs, rabbits and macaques. Virology 2014, 456–457, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.A.; Bauer, M.; Manolova, V.; Muntwiler, S.; Saudan, P.; Bachmann, M.F. Cutting Edge: Limited Specialization of Dendritic Cell Subsets for MHC Class II-Associated Presentation of Viral Particles. J. Immunol. 2010, 184, 26–29. [Google Scholar] [CrossRef]
- Dorner, B.G.; Dorner, M.B.; Zhou, X.; Opitz, C.; Mora, A.; Güttler, S.; Hutloff, A.; Mages, H.W.; Ranke, K.; Schaefer, M.; et al. Selective Expression of the Chemokine Receptor XCR1 on Cross-presenting Dendritic Cells Determines Cooperation with CD8+ T Cells. Immunity 2009, 31, 823–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wylie, B.; Read, J.; Buzzai, A.C.; Wagner, T.; Troy, N.; Syn, G.; Stone, S.R.; Foley, B.; Bosco, A.; Cruickshank, M.N.; et al. CD8+XCR1neg Dendritic Cells Express High Levels of Toll-Like Receptor 5 and a Unique Complement of Endocytic Receptors. Front. Immunol. 2019, 9, 2990. [Google Scholar] [CrossRef] [PubMed]
- Esparza, J. A brief history of the global effort to develop a preventive HIV vaccine. Vaccine 2013, 31, 3502–3518. [Google Scholar] [CrossRef] [Green Version]
- Temchura, V.V.; Tenbusch, M.; Nchinda, G.; Nabi, G.; Tippler, B.; Zelenyuk, M.; Wildner, O.; Überla, K.; Kuate, S. Enhancement of immunostimulatory properties of exosomal vaccines by incorporation of fusion-competent G protein of vesicular stomatitis virus. Vaccine 2008, 26, 3662–3672. [Google Scholar] [CrossRef]







| Abbreviations | Envelope Proteins | Structural Proteins |
|---|---|---|
| VLP | - | HIV-Gag/Pol 7 |
| VLP-KFΔ | KFΔ 1 | HIV-Gag/Pol 7 |
| Env-VLP | HIV-Env 2 | HIV-Gag/Pol 7 |
| Env-VLP-KFΔ | HIV-Env 3; KFΔ 4 | HIV-Gag/Pol 7 |
| HEL-VLP | HEL 5 | HIV-Gag/Pol 7 |
| HEL-VLP-KFΔ | HEL 6; KFΔ 4 | HIV-Gag/Pol 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnowski, C.; Kadzioch, N.; Damm, D.; Yan, H.; Temchura, V. Advantages and Limitations of Integrated Flagellin Adjuvants for HIV-Based Nanoparticle B-Cell Vaccines. Pharmaceutics 2019, 11, 204. https://doi.org/10.3390/pharmaceutics11050204
Barnowski C, Kadzioch N, Damm D, Yan H, Temchura V. Advantages and Limitations of Integrated Flagellin Adjuvants for HIV-Based Nanoparticle B-Cell Vaccines. Pharmaceutics. 2019; 11(5):204. https://doi.org/10.3390/pharmaceutics11050204
Chicago/Turabian StyleBarnowski, Cornelia, Nicole Kadzioch, Dominik Damm, Huimin Yan, and Vladimir Temchura. 2019. "Advantages and Limitations of Integrated Flagellin Adjuvants for HIV-Based Nanoparticle B-Cell Vaccines" Pharmaceutics 11, no. 5: 204. https://doi.org/10.3390/pharmaceutics11050204
APA StyleBarnowski, C., Kadzioch, N., Damm, D., Yan, H., & Temchura, V. (2019). Advantages and Limitations of Integrated Flagellin Adjuvants for HIV-Based Nanoparticle B-Cell Vaccines. Pharmaceutics, 11(5), 204. https://doi.org/10.3390/pharmaceutics11050204