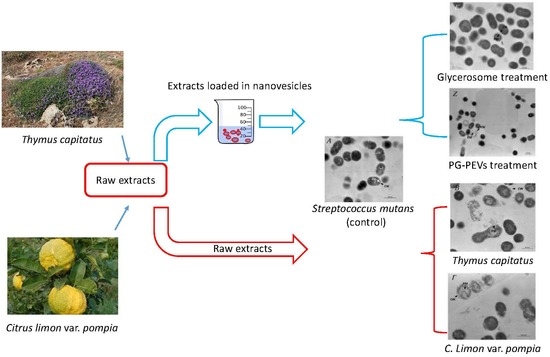
Antimicrobial Effect of Thymus capitatus and Citrus limon var. pompia as Raw Extracts and Nanovesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection
2.2. Extraction Procedures
2.3. Identification of TC and CLP Components
2.3.1. GC-MS Analysis of TC Essential Oil
2.3.2. GC-FID Analysis of TC Essential Oil
2.3.3. Targeted LC-MS/MS Analysis of CLP Extract
2.4. Vesicle Preparation and Characterization
2.4.1. Vesicle Preparation
2.4.2. Vesicle Characterization
2.5. Cytotoxicity Assay
2.6. Antimicrobial Assays
2.6.1. Preparation of the Microbial Inocula
2.6.2. Disc Diffusion Method
2.6.3. Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), and Minimum Fungicidal Concentration (MFC)
2.6.4. Microbial Killing Rates by Time-Kill Assay
2.7. Transmission Electron Microscopy
3. Results
3.1. Chemical Composition
3.2. Vesicles Characterization
3.3. Cytotoxicity Assay
3.4. Disc Diffusion Method
3.5. MIC-MBC/MFC
3.6. Time-Kill Assay
3.7. Transmission Electron Microscopy (TEM)
3.7.1. Untreated S. mutans
3.7.2. Treated S. mutans
3.7.3. Untreated C. albicans
3.7.4. Treated C. albicans
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marsh, P.D. Sugar, fluoride, pH and microbial homeostasis in dental plaque. Proc. Finn. Dent. Soc. 1991, 87, 515–525. [Google Scholar]
- Marsh, P.D. Plaque as a biofilm: Pharmacological principles of drug delivery and action in the sub- and supragingival environment. Oral Dis. 2003, 9 (Suppl. 1), 16–22. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.-H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef] [PubMed]
- Pinna, R.; Campus, G.; Cumbo, E.; Mura, I.; Milia, E. Xerostomia induced by radiotherapy: An overview of the physiopathology, clinical evidence, and management of the oral damage. Ther. Clin. Risk Manag. 2015, 11, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Cagetti, M.G.; Campus, G.; Milia, E.; Lingström, P. A systematic review on fluoridated food in caries prevention. Acta Odontol. Scand. 2013, 71, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Milia, E.; Castelli, G.; Bortone, A.; Sotgiu, G.; Manunta, A.; Pinna, R.; Gallina, G. Short-term response of three resin-based materials as desensitizing agents under oral environmental exposure. Acta Odontol. Scand. 2013, 71, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Controlling the oral biofilm with antimicrobials. J. Dent. 2010, 38, S11–S15. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; ISBN 9789241564748. [Google Scholar]
- Waikedre, J.; Dugay, A.; Barrachina, I.; Herrenknecht, C.; Cabalion, P.; Fournet, A. Chemical Composition and Antimicrobial Activity of the Essential Oils from New Caledonian Citrus macroptera and Citrus hystrix. Chem. Biodivers. 2010, 7, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Verkaik, M.J.; Busscher, H.J.; Jager, D.; Slomp, A.M.; Abbas, F.; van der Mei, H.C. Efficacy of natural antimicrobials in toothpaste formulations against oral biofilms in vitro. J. Dent. 2011, 39, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Hooper, S.J.; Lewis, M.A.O.; Wilson, M.J.; Williams, D.W. Antimicrobial activity of Citrox® bioflavonoid preparations against oral microorganisms. Br. Dent. J. 2011, 210, E22. [Google Scholar] [CrossRef] [PubMed]
- Chaillot, J.; Tebbji, F.; Remmal, A.; Boone, C.; Brown, G.W.; Bellaoui, M.; Sellam, A. The Monoterpene Carvacrol Generates Endoplasmic Reticulum Stress in the Pathogenic Fungus Candida albicans. Antimicrob. Agents Chemother. 2015, 59, 4584–4592. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Khan, M.; Ahmad, J.; Wahab, R.; Abd-Elkader, O.H.; Musarrat, J.; Alkhathlan, H.Z.; Al-Kedhairy, A.A. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express 2017, 7, 49. [Google Scholar] [CrossRef]
- Silva, P.; Bonifácio, B.; Ramos, M.; Negri, K.; Maria Bauab, T.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2013, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Saraf, S.; Jain, A.; Hurkat, P.; Jain, S.K. Topotecan Liposomes: A Visit from a Molecular to a Therapeutic Platform. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 401–432. [Google Scholar] [CrossRef]
- Matougui, N.; Boge, L.; Groo, A.-C.; Umerska, A.; Ringstad, L.; Bysell, H.; Saulnier, P. Lipid-based nanoformulations for peptide delivery. Int. J. Pharm. 2016, 502, 80–97. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wang, X.; Sun, L.; Zhou, Z.; Lu, J.; Zheng, Y. Encapsulation of low lipophilic and slightly water-soluble dihydroartemisinin in PLGA nanoparticles with phospholipid to enhance encapsulation efficiency and in vitro bioactivity. J. Microencapsul. 2016, 33, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manca, M.L.; Caddeo, C.; Maxia, A.; Murgia, S.; Pons, R.; Demurtas, D.; Pando, D.; Falconieri, D.; Peris, J.E.; et al. Faceted phospholipid vesicles tailored for the delivery of Santolina insularis essential oil to the skin. Coll. Surf. B Biointerfaces 2015, 132, 185–193. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Marongiu, F.; Caddeo, C.; Castangia, I.; Petretto, G.L.; Pintore, G.; Sarais, G.; D’Hallewin, G.; Zaru, M.; et al. Chemical characterization of Citrus limon var. pompia and incorporation in phospholipid vesicles for skin delivery. Int. J. Pharm. 2016, 506, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Cruciani, S.; Santaniello, S.; Garroni, G.; Fadda, A.; Balzano, F.; Bellu, E.; Sarais, G.; Fais, G.; Mulas, M.; Maioli, M.; et al. Myrtus Polyphenols, from Antioxidants to Anti-Inflammatory Molecules: Exploring a Network Involving Cytochromes P450 and Vitamin D. Molecules 2019, 24, 1515. [Google Scholar] [CrossRef]
- Ferhi, S.; Santaniello, S.; Zerizer, S.; Cruciani, S.; Fadda, A.; Sanna, D.; Dore, A.; Maioli, M.; D’hallewin, G.; Ferhi, S.; et al. Total Phenols from Grape Leaves Counteract Cell Proliferation and Modulate Apoptosis-Related Gene Expression in MCF-7 and HepG2 Human Cancer Cell Lines. Molecules 2019, 24, 612. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C.; Mohammed, M.J.; Stelling, J.; O’Brien, T.; Williams, R. Ability of laboratories to detect emerging antimicrobial resistance: Proficiency Testing and Quality Control results from the World Health Organization’s External Quality Assurance System for Antimicrobial Susceptibility Testing. J. Clin. Microbiol. 2001, 39, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Hacek, D.M.; Dressel, D.C.; Peterson, L.R. Highly reproducible bactericidal activity test results by using a modified National Committee for Clinical Laboratory Standards broth macrodilution technique. J. Clin. Microbiol. 1999, 37, 1881–1884. [Google Scholar]
- Juliano, C.; Demurtas, C.; Piu, L. In vitro study on the anticandidal activity of Melaleuca alternifolia (tea tree) essential oil combined with chitosan. Flavour Fragr. J. 2008, 23, 227–231. [Google Scholar] [CrossRef]
- Milia, E.; Lallai, M.R.; García-Godoy, F. In vivo effect of a self-etching primer on dentin. Am. J. Dent. 1999, 12, 167–171. [Google Scholar] [PubMed]
- Milia, E.; Cumbo, E.; Cardoso, R.J.A.; Gallina, G. Current dental adhesives systems. A narrative review. Curr. Pharm. Des. 2012, 18, 5542–5552. [Google Scholar] [CrossRef]
- Milia, E.; Pinna, R.; Castelli, G.; Bortone, A.; Marceddu, S.; Garcia-Godoy, F.; Gallina, G. TEM morphological characterization of a one-step self-etching system applied clinically to human caries-affected dentin and deep sound dentin. Am. J. Dent. 2012, 25, 321–326. [Google Scholar]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef]
- National Institute of Health Consensus Development Panel. National Institutes of Health Consensus Development Conference statement. Diagnosis and management of dental caries throughout life. J. Am. Dent. Assoc. 2001, 132, 1153–1161. [Google Scholar] [CrossRef]
- Marinho, V.C.; Chong, L.Y.; Worthington, H.V.; Walsh, T. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2016, 7, CD002284. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Prim. 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Microbial Ecology of Dental Plaque and its Significance in Health and Disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef]
- Rmaile, A. Mechanical Properties and Disruption of Dental Biofilms. Ph.D. Thesis, University of Southampton, Southampton, UK, 2013. [Google Scholar]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef]
- Jeon, J.-G.; Rosalen, P.L.; Falsetta, M.L.; Koo, H. Natural Products in Caries Research: Current (Limited) Knowledge, Challenges and Future Perspective. Caries Res. 2011, 45, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Norrby, S.R.; Nord, C.E.; Finch, R.; European Society of Clinical Microbiology and Infectious Diseases. Lack of development of new antimicrobial drugs: A potential serious threat to public health. Lancet Infect. Dis. 2005, 5, 115–119. [Google Scholar] [CrossRef]
- Valeriani, F.; Protano, C.; Gianfranceschi, G.; Cozza, P.; Campanella, V.; Liguori, G.; Vitali, M.; Divizia, M.; Romano Spica, V. Infection control in healthcare settings: Perspectives for mfDNA analysis in monitoring sanitation procedures. BMC Infect. Dis. 2016, 16, 394. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Campanella, V.; Syed, J.; Santacroce, L.; Saini, R.; Ballini, A.; Inchingolo, F. Oral probiotics influence oral and respiratory tract infections in pediatric population: A randomized double-blinded placebo-controlled pilot study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8034–8041. [Google Scholar] [CrossRef] [PubMed]
- Sherry, M.; Charcosset, C.; Fessi, H.; Greige-Gerges, H. Essential oils encapsulated in liposomes: A review. J. Liposome Res. 2013, 23, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Sales, O.D.; Valenti, D.; Saurí, A.R.; Fadda, A.M.; Manconi, M. Inhibition of skin inflammation in mice by diclofenac in vesicular carriers: Liposomes, ethosomes and PEVs. Int. J. Pharm. 2013, 443, 128–136. [Google Scholar] [CrossRef]
- Smith, R.L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Hall, R.L.; et al. A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: Essential oils. Food Chem. Toxicol. 2005, 43, 345–363. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrimsson, O.; Thormar, H. In Vitro Killing of Candida albicans by Fatty Acids and Monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Mai, S.; Mauger, M.T.; Niu, L.; Barnes, J.B.; Kao, S.; Bergeron, B.E.; Ling, J.; Tay, F.R. Potential applications of antimicrobial peptides and their mimics in combating caries and pulpal infections. Acta Biomater. 2017, 49, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Slump, R.A.; Steging, G.; Smid, E.J. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J. Food Prot. 2000, 63, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.F.; Storm, G.; et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P.; Angioni, A. Characterization of the volatile constituents in the essential oil of Pistacia lentiscus l. from different origins and its antifungal and antioxidant activity. J. Agric. Food Chem. 2007, 55, 7093–7098. [Google Scholar] [CrossRef]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef]
- Pieri, F.A.; de Castro Souza, M.C.; Vermelho, L.L.R.; Vermelho, M.L.R.; Perciano, P.G.; Vargas, F.S.; Borges, A.P.B.; da Veiga-Junior, V.F.; Moreira, M.A.S. Use of β-caryophyllene to combat bacterial dental plaque formation in dogs. BMC Vet. Res. 2016, 12, 216. [Google Scholar] [CrossRef]
- Malmsten, M. Interactions of Antimicrobial Peptides with Bacterial Membranes and Membrane Components. Curr. Top. Med. Chem. 2016, 16, 16–24. [Google Scholar] [CrossRef]
- Ultee, A.; Gorris, L.G.; Smid, E.J. Bactericidal activity of carvacrol towards the food-borne pathogen Bacillus cereus. J. Appl. Microbiol. 1998, 85, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M.N.; Carezzano, M.E.; Oliva, M.M.; Demo, M.S.; Pizzolitto, R.P.; Zunino, M.P.; Zygadlo, J.A.; Dambolena, J.S. In vitro activity of natural phenolic compounds against fluconazole-resistant Candida species: A quantitative structure-activity relationship analysis. J. Appl. Microbiol. 2014, 116, 795–804. [Google Scholar] [CrossRef] [PubMed]
- De Galvão, L.C.C.; Furletti, V.F.; Bersan, S.M.F.; Da Cunha, M.G.; Ruiz, A.L.T.G.; Carvalho, J.E.D.; Sartoratto, A.; Rehder, V.L.G.; Figueira, G.M.; Teixeira Duarte, M.C.; et al. Antimicrobial activity of essential oils against Streptococcus mutans and their antiproliferative effects. Evid. Based Complement. Altern. Med. 2012, 2012, 751435. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry and multibeneficial bioactivities of carvacrol (4-isopropyl-2-methylphenol), a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K.; Calandra, T.F.; Edwards, J.E., Jr.; Filler, S.G.; Fisher, J.F.; Kullberg, B.; Ostrosky-Zeichner, L.; et al. Clinical Practice Guidelines for the Management of Candidiasis: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef] [PubMed]
- Stana, A.; Vodnar, D.C.; Tamaian, R.; Pîrnău, A.; Vlase, L.; Ionuț, I.; Oniga, O.; Tiperciuc, B. Design, synthesis and antifungal activity evaluation of new thiazolin-4-ones as potential lanosterol 14α-demethylase inhibitors. Int. J. Mol. Sci. 2017, 18, 177. [Google Scholar] [CrossRef] [PubMed]
- Akins, R.A. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med. Mycol. 2005, 43, 285–318. [Google Scholar] [CrossRef]
- Perlin, D.S. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 2007, 10, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory Circuitry Governing Fungal Development, Drug Resistance, and Disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef]
- Umerska, A.; Cassisa, V.; Matougui, N.; Joly-Guillou, M.-L.; Eveillard, M.; Saulnier, P. Antibacterial action of lipid nanocapsules containing fatty acids or monoglycerides as co-surfactants. Eur. J. Pharm. Biopharm. 2016, 108, 100–110. [Google Scholar] [CrossRef] [PubMed]






| Sample | Soy Lecithin (mg) | Essential Oil/Extract (mg) | Water (mL) | Glycerol (mL) | PG (mL) |
|---|---|---|---|---|---|
| TC liposomes | 60 | 10 | 1.0 | -- | -- |
| TC glycerosomes | 60 | 10 | 0.5 | 0.5 | -- |
| TC PG-PEVs | 60 | 10 | 0.5 | -- | 0.5 |
| CLP liposomes | 60 | 10 | 1.0 | -- | -- |
| CLP glycerosomes | 60 | 10 | 0.5 | 0.5 | -- |
| CLP PG-PEVs | 60 | 10 | 0.5 | -- | 0.5 |
| Main Components of TC Essential Oil | A% | RI |
|---|---|---|
| p-cymene | 1.1 | 1022.9 |
| limonene | 0.2 | 1026.5 |
| 1,8-cineole | 0.6 | 1028.8 |
| γ-terpinene | 0.4 | 1066.5 |
| linalool | 1.6 | 1100.9 |
| camphor | 0.1 | 1142.5 |
| borneol | 0.7 | 1165.1 |
| terpinen-4-ol | 1.1 | 1176.9 |
| α-terpineol | 0.2 | 1192.7 |
| Thymol | 0.5 | 1294.6 |
| carvacrol | 90.1 | 1306.5 |
| carvacrol acetate | 0.1 | 1375.3 |
| cariophyllene | 1.0 | 1419.3 |
| cariophyllene oxide | 1.3 | 1586.2 |
| Sample | MD (nm) | PI | ZP (mV) |
|---|---|---|---|
| TC liposomes | 86 ± 12 | 0.25 | –72 ± 12 |
| TC glycerosomes | 479 ± 40 | 0.23 | –49 ± 3 |
| TC PG-PEVs | 337 ± 45 | 0.24 | –50 ± 1 |
| CLP liposomes | 137 ± 16 | 0.26 | –43 ± 4 |
| CLP glycerosomes | 180 ± 16 | 0.30 | –51 ± 9 |
| CLP PG-PEVs | 218 ± 28 | 0.30 | –65 ± 5 |
| TC Essential Oil | S. mutans | C. albicans | ||
|---|---|---|---|---|
| Mean ± SD | 95%CI | Mean ± SD | 95%CI | |
| Raw oil | 12 ± 0 | 12–12 | 12 ± 0.5 | 10.76–13.24 |
| Liposomes | 13 ± 0 | 13–13 | 0 ± 0 | 0–0 |
| Glycerosomes | 11 ± 0 | 11–11 | 11.83 ± 0.29 | 11.12–12.6 |
| PG-PEVs | 0 ± 0 | 0–0 | 9 ± 1.73 | 4.69–13.30 |
| Gentamicin | 14 ± 0 | 14–14 | - | - |
| Ketoconazole | - | - | 11.33 ± 0.58 | 9.89–12.77 |
| CLP Extract | S. mutans | C. albicans | ||
|---|---|---|---|---|
| Mean ± SD | 95%CI | Mean ± SD | 95%CI | |
| Raw extract | 0 ± 0 | 0–0 | 0 ± 0 | 0–0 |
| Liposomes | 8.97 ± 0.58 | 8.82–9.11 | 0 ± 0 | 0–0 |
| Glycerosomes | 14 ± 0 | 14.0–14.0 | 0 ± 0 | 0–0 |
| PG-PEVs | 10 ± 0 | 10–10 | 0 ± 0 | 0–0 |
| Strain | Raw Essential Oil | Liposomes | Glycerosomes | PG-PEVs | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC/MFC | MIC | MBC/MFC | MIC | MBC/MFC | MIC | MBC/MFC | |
| S. mutans | 0.5 | 0.5 | >2.5 | >2.5 | <0.078 | <0.078 | <0.078 | <0.078 |
| C. albicans | 0.25 | 0.5 | >2.5 | >2.5 | >2.5 | >2.5 | >2.5 | >2.5 |
| Strain | Raw Essential Oil | Liposomes | Glycerosomes | PG-PEVs | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC/MFC | MIC | MBC/MFC | MIC | MBC/MFC | MIC | MBC/MFC | |
| S. mutans | 0.625 | 0.625 | >2.5 | >2.5 | <0.078 | <0.078 | <0.078 | <0.078 |
| C. albicans | 0.625 | 0.625 | >2.5 | >2.5 | >2.5 | >2.5 | >2.5 | >2.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinna, R.; Filigheddu, E.; Juliano, C.; Palmieri, A.; Manconi, M.; D’hallewin, G.; Petretto, G.; Maioli, M.; Caddeo, C.; Manca, M.L.; et al. Antimicrobial Effect of Thymus capitatus and Citrus limon var. pompia as Raw Extracts and Nanovesicles. Pharmaceutics 2019, 11, 234. https://doi.org/10.3390/pharmaceutics11050234
Pinna R, Filigheddu E, Juliano C, Palmieri A, Manconi M, D’hallewin G, Petretto G, Maioli M, Caddeo C, Manca ML, et al. Antimicrobial Effect of Thymus capitatus and Citrus limon var. pompia as Raw Extracts and Nanovesicles. Pharmaceutics. 2019; 11(5):234. https://doi.org/10.3390/pharmaceutics11050234
Chicago/Turabian StylePinna, Roberto, Enrica Filigheddu, Claudia Juliano, Alessandra Palmieri, Maria Manconi, Guy D’hallewin, Giacomo Petretto, Margherita Maioli, Carla Caddeo, Maria Letizia Manca, and et al. 2019. "Antimicrobial Effect of Thymus capitatus and Citrus limon var. pompia as Raw Extracts and Nanovesicles" Pharmaceutics 11, no. 5: 234. https://doi.org/10.3390/pharmaceutics11050234
APA StylePinna, R., Filigheddu, E., Juliano, C., Palmieri, A., Manconi, M., D’hallewin, G., Petretto, G., Maioli, M., Caddeo, C., Manca, M. L., Solinas, G., Bortone, A., Campanella, V., & Milia, E. (2019). Antimicrobial Effect of Thymus capitatus and Citrus limon var. pompia as Raw Extracts and Nanovesicles. Pharmaceutics, 11(5), 234. https://doi.org/10.3390/pharmaceutics11050234