Nanostructured Lipid Carrier Gel for the Dermal Application of Lidocaine: Comparison of Skin Penetration Testing Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the NLC
2.2.2. Laser Diffraction
2.2.3. Rheological Measurements
2.2.4. Microscopic Examinations
2.2.5. pH Measurements
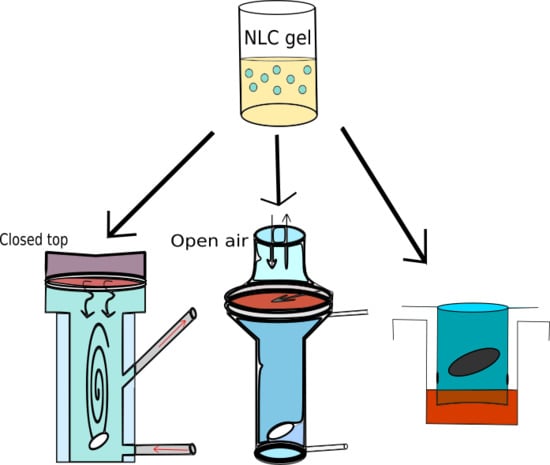
2.2.6. Drug Diffusion and Penetration Studies
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Particle Size Analysis
3.2. Microscopic Study
3.3. Rheological Studies
3.4. pH Measurement
3.5. Drug Release, Diffusion and Penetration Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shah, V.P.; Yacobi, A.; Rădulescu, F.Ş.; Miron, D.S.; Lane, M.E. A science based approach to topical drug classification system (TCS). Int. J. Pharm. 2015, 491, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Drug Penetration Into/Through the Skin; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-53268-3. [Google Scholar]
- Ruela, A.L.M.; Perissinato, A.G.; Lino, M.E.D.S.; Mudrik, P.S.; Pereira, G.R. Evaluation of skin absorption of drugs from topical and transdermal formulations. Br. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef] [Green Version]
- OECD. Guidance Notes on Dermal Absorption, Series on Testing and Assessment No. 156; OECD: Paris, France, 2011. [Google Scholar]
- OECD. Test Guideline 428: Skin Absorption: In vitro Method; OECD: Paris, France, 2004. [Google Scholar]
- OECD. Test Guideline 427: Skin Absorption: In vivo Method; OECD: Paris, France, 2004. [Google Scholar]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies; OECD: Paris, France, 2004. [Google Scholar]
- European Food Safety Authority (EFSA); Buist, H.; Craig, P.; Dewhurst, I.; Hougaard Bennekou, S.; Kneuer, C.; Machera, K.; Pieper, C.; Court Marques, D.; Guillot, G.; et al. Guidance on dermal absorption. EFSA J. 2017, 15, 4873. [Google Scholar] [CrossRef]
- Shah, V.P.; Elkins, J.; Shaw, S.; Hanson, R. In Vitro Release: Comparative Evaluation of Vertical Diffusion Cell System and Automated Procedure: RESEARCH ARTICLE. Pharm. Dev. Technol. 2003, 8, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Hauck, W.W.; Shah, V.P.; Shaw, S.W.; Ueda, C.T. Reliability and Reproducibility of Vertical Diffusion Cells for Determining Release Rates from Semisolid Dosage Forms. Pharm. Res. 2007, 24, 2018–2024. [Google Scholar] [CrossRef]
- Franz, T.J. Percutaneous Absorption. On the Relevance of in Vitro Data. J. Invest. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Bakonyi, M.; Gácsi, A.; Berkó, S.; Kovács, A.; Csányi, E. Stratum corneum lipid liposomes for investigating skin penetration enhancer effects. RSC Adv. 2018, 8, 27464–27469. [Google Scholar] [CrossRef] [Green Version]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In vitro skin models as a tool in optimization of drug formulation. Eur. J. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Intarakumhaeng, R.; Alsheddi, L.; Wanasathop, A.; Shi, Z.; Li, S.K. Skin Permeation of Urea Under Finite Dose Condition. J. Pharm. Sci. 2019, 108, 987–995. [Google Scholar] [CrossRef]
- Trombino, S.; Russo, R.; Mellace, S.; Varano, G.P.; Laganà, A.S.; Marcucci, F.; Cassano, R. Solid lipid nanoparticles made of trehalose monooleate for cyclosporin-A topic release. J. Drug Deliv. Sci. Technol. 2019, 49, 563–569. [Google Scholar] [CrossRef]
- Soriano-Ruiz, J.L.; Suñer-Carbó, J.; Calpena-Campmany, A.C.; Bozal-de Febrer, N.; Halbaut-Bellowa, L.; Boix-Montañés, A.; Souto, E.B.; Clares-Naveros, B. Clotrimazole multiple W/O/W emulsion as anticandidal agent: Characterization and evaluation on skin and mucosae. Coll. Surf. B Biointerfaces 2019, 175, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lane, M.E.; Hadgraft, J.; Heinrich, M.; Chen, T.; Lian, G.; Sinko, B. A comparison of the in vitro permeation of niacinamide in mammalian skin and in the Parallel Artificial Membrane Permeation Assay (PAMPA) model. Int. J. Pharm. 2019, 556, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Castelli, F.; Sarpietro, M. Differential Scanning Calorimetry Analyses of Idebenone-Loaded Solid Lipid Nanoparticles Interactions with a Model of Bio-Membrane: A Comparison with In Vitro Skin Permeation Data. Pharmaceuticals 2018, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Dahlizar, S.; Futaki, M.; Okada, A.; Yatomi, C.; Todo, H.; Sugibayashi, K. Combined Use of N-Palmitoyl-Glycine-Histidine Gel and Several Penetration Enhancers on the Skin Permeation and Concentration of Metronidazole. Pharmaceutics 2018, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Balázs, B. Investigation of the Skin Barrier Function and Transdermal Drug Delivery Techniques. Ph.D. Thesis, Unviersity of Szeged, Szeged, Hungary, 2016. [Google Scholar]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative evaluation of rivastigmine permeation from a transdermal system in the Franz cell using synthetic membranes and pig ear skin with in vivo-in vitro correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of skin permeation by chemical compounds using the artificial membrane, Strat-MTM. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Kim, M.-H.; Park, J.-H.; Lee, J.-Y.; Cho, H.-J.; Yoon, I.-S.; Kim, D.-D. Microemulsion-based hydrogels for enhancing epidermal/dermal deposition of topically administered 20(S)-protopanaxadiol: In vitro and in vivo evaluation studies. J. Ginseng Res. 2018, 42, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, M.; Berkó, S.; Kovács, A.; Budai-Szűcs, M.; Kis, N.; Erős, G.; Csóka, I.; Csányi, E. Application of quality by design principles in the development and evaluation of semisolid drug carrier systems for the transdermal delivery of lidocaine. J. Drug Deliv. Sci. Technol. 2018, 44, 136–145. [Google Scholar] [CrossRef]
- Sütő, B.; Berkó, S.; Kozma, G.; Kukovecz, Á.; Budai-Szűcs, M.; Erős, G.; Kemény, L.; Sztojkov-Ivanov, A.; Gaspar, R.; Csányi, E. Development of ibuprofen-loaded nanostructured lipid carrier-based gels: Characterization and investigation of in vitro and in vivo penetration through the skin. Int. J. Nanomed. 2016, 11, 1201–1212. [Google Scholar]
- Sinkó, B.; Garrigues, T.M.; Balogh, G.T.; Nagy, Z.K.; Tsinman, O.; Avdeef, A.; Takács-Novák, K. Skin–PAMPA: A new method for fast prediction of skin penetration. Eur. J. Pharm. Sci. 2012, 45, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Köllmer, M.; Mossahebi, P.; Sacharow, E.; Gorissen, S.; Gräfe, N.; Evers, D.-H.; Herbig, M.E. Investigation of the Compatibility of the Skin PAMPA Model with Topical Formulation and Acceptor Media Additives Using Different Assay Setups. AAPS PharmSciTech 2019, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Vizserálek, G.; Berkó, S.; Tóth, G.; Balogh, R.; Budai-Szűcs, M.; Csányi, E.; Sinkó, B.; Takács-Novák, K. Permeability test for transdermal and local therapeutic patches using Skin PAMPA method. Eur. J. Pharm. Sci. 2015, 76, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Sinkó, B.; Vizserálek, G.; Takács-Novák, K. Skin PAMPA: Application in practice. ADMET DMPK 2015, 2, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Patel, A.; Sinko, B.; Bell, M.; Wibawa, J.; Hadgraft, J.; Lane, M.E. A comparative study of the in vitro permeation of ibuprofen in mammalian skin, the PAMPA model and silicone membrane. Int. J. Pharm. 2016, 505, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakonyi, M.; Gácsi, A.; Kovács, A.; Szűcs, M.-B.; Berkó, S.; Csányi, E. Following-up skin penetration of lidocaine from different vehicles by Raman spectroscopic mapping. J. Pharm. Biomed. Anal. 2018, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Sarpietro, M.G.; Bonina, F.; Castelli, F.; Zammataro, M.; Chiechio, S. Development, Characterization, and In Vitro and In Vivo Evaluation of Benzocaine- and Lidocaine-Loaded Nanostructrured Lipid Carriers. J. Pharma. Sci. 2011, 100, 1892–1899. [Google Scholar] [CrossRef]
- Ribeiro, L.N.M.; Breitkreitz, M.C.; Guilherme, V.A.; da Silva, G.H.R.; Couto, V.M.; Castro, S.R.; de Paula, B.O.; Machado, D.; de Paula, E. Natural lipids-based NLC containing lidocaine: From pre-formulation to in vivo studies. Eur. J. Pharm. Sci. 2017, 106, 102–112. [Google Scholar] [CrossRef]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, 131–155. [Google Scholar] [CrossRef]
- Rincón, M.; Calpena, A.; Fabrega, M.-J.; Garduño-Ramírez, M.; Espina, M.; Rodríguez-Lagunas, M.; García, M.; Abrego, G. Development of Pranoprofen Loaded Nanostructured Lipid Carriers to Improve Its Release and Therapeutic Efficacy in Skin Inflammatory Disorders. Nanomaterials 2018, 8, 1022. [Google Scholar] [CrossRef]
- You, P.; Yuan, R.; Chen, C. Design and evaluation of lidocaine- and prilocaine-coloaded nanoparticulate drug delivery systems for topical anesthetic analgesic therapy: A comparison between solid lipid nanoparticles and nanostructured lipid carriers. Drug Design Dev. Ther. 2017, 11, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.; Cai, Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Draft Guideline on Quality and Equivalence of Topical Products; EMA/CHMP/QWP/708282/2018; European Medicines Agency: Amsterdam, The Netherlands, 2018.




| LID-NLC | Blank NLC | LID-NLC Gel | Blank NLC-Gel |
|---|---|---|---|
| Apifil 11.8% | Apifil 11.8% | LID-NLC 89% | Blank NLC 89% |
| Cremophor RH 60.8% | Cremophor RH 60.8% | Glycerin 8% | Glycerin 8% |
| Miglyol 812 N 5% | Miglyol 812 N 5% | Methocel E4M 3% | Methocel E4M 3% |
| Purified water 69.2% | Purified water 75.2% | ||
| Lidocaine 6% |
| Device | Hanson | Logan | Skin-PAMPA |
|---|---|---|---|
| Membrane | Cellulose acetate | Cellulose acetate | Skin-PAMPA membrane |
| Strat-M® | Strat-M® | ||
| HSE | HSE |
| Day | NLC Dispersion | d(0.1) µm | d(0.5) µm | d(0.9) µm | Span |
|---|---|---|---|---|---|
| 1st day | Blank-NLC, room temperature | 0.066 | 0.131 | 0.320 | 1.950 |
| Blank-NLC, cooled | 0.066 | 0.130 | 0.302 | 1.821 | |
| LID-NLC, room temperature | 0.069 | 0.098 | 0.145 | 0.787 | |
| LID-NLC, cooled | 0.069 | 0.098 | 0.146 | 0.790 | |
| 2nd day | Blank-NLC, room temperature | 0.065 | 0.127 | 0.266 | 1.584 |
| Blank-NLC, cooled | 0.065 | 0.127 | 0.266 | 1.587 | |
| LID-NLC, room temperature | 0.068 | 0.097 | 0.144 | 0.782 | |
| LID-NLC, cooled | 0.073 | 0.114 | 0.259 | 1.625 | |
| 3rd day | Blank-NLC, room temperature | 0.065 | 0.126 | 0.258 | 1.542 |
| Blank-NLC, cooled | 0.065 | 0.126 | 0.262 | 1.564 | |
| LID-NLC, room temperature | 0.069 | 0.098 | 0.146 | 0.791 | |
| LID-NLC, cooled | 0.079 | 0.130 | 0.300 | 1.698 | |
| 4th day | Blank-NLC, room temperature | 0.065 | 0.128 | 0.289 | 1.746 |
| Blank-NLC, cooled | 0.065 | 0.128 | 0.298 | 1.814 | |
| LID-NLC, room temperature | 0.090 | 0.163 | 41.749 | 255.201 | |
| LID-NLC, cooled | - | - | - | - |
| Time of Experiment | Cellulose | Strat-M® | HSE | Skin-PAMPA | |||
|---|---|---|---|---|---|---|---|
| Hanson | Logan | Hanson | Logan | Hanson | Logan | ||
| 6 h (µg/cm2) | 3808.82 ± 448.91 | 3355.40 ± 320.70 | 304.20 ± 113.40 | 514.89 ± 209.69 | 1778.25 ± 483.81 | 670.85 ± 189.05 | 696.32 ± 20.50 |
| 6 h (%) | 41.35 ± 18.8 | 27.73 ± 0.83 | 3.45 ± 1.21 | 7.72 ± 2.89 | 11.61 ± 2.95 | 8.785 ± 2.03 | 13.93 ± 0.41 |
| 24 h (µg/cm2) | 8014.05 ± 471.89 | 6878.13 ± 1172.21 | 1079.27 ± 304.36 | 1847.08 ± 335.19 | 3094.60 ± 829.73 | 1222.69 ± 358.70 | - |
| 24 h (%) | 70.40 ± 13.72 | 58.66 ± 4.28 | 12.96 ± 3.52 | 27.89 ± 5.17 | 19.44 ± 5.05 | 16.00 ± 3.81 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zsikó, S.; Cutcher, K.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Baki, G.; Csányi, E.; Berkó, S. Nanostructured Lipid Carrier Gel for the Dermal Application of Lidocaine: Comparison of Skin Penetration Testing Methods. Pharmaceutics 2019, 11, 310. https://doi.org/10.3390/pharmaceutics11070310
Zsikó S, Cutcher K, Kovács A, Budai-Szűcs M, Gácsi A, Baki G, Csányi E, Berkó S. Nanostructured Lipid Carrier Gel for the Dermal Application of Lidocaine: Comparison of Skin Penetration Testing Methods. Pharmaceutics. 2019; 11(7):310. https://doi.org/10.3390/pharmaceutics11070310
Chicago/Turabian StyleZsikó, Stella, Kendra Cutcher, Anita Kovács, Mária Budai-Szűcs, Attila Gácsi, Gabriella Baki, Erzsébet Csányi, and Szilvia Berkó. 2019. "Nanostructured Lipid Carrier Gel for the Dermal Application of Lidocaine: Comparison of Skin Penetration Testing Methods" Pharmaceutics 11, no. 7: 310. https://doi.org/10.3390/pharmaceutics11070310
APA StyleZsikó, S., Cutcher, K., Kovács, A., Budai-Szűcs, M., Gácsi, A., Baki, G., Csányi, E., & Berkó, S. (2019). Nanostructured Lipid Carrier Gel for the Dermal Application of Lidocaine: Comparison of Skin Penetration Testing Methods. Pharmaceutics, 11(7), 310. https://doi.org/10.3390/pharmaceutics11070310