Evolution from Covalent to Self-Assembled PAMAM-Based Dendrimers as Nanovectors for siRNA Delivery in Cancer by Coupled In Silico-Experimental Studies. Part I: Covalent siRNA Nanocarriers
Abstract
:1. RNA Interference and Challenges in Small Interference RNA Therapeutics
2. Role of Dendrimers as siRNA Nanocarriers
3. Structurally Flexible PAMAM Dendrimers for Safe, Efficient and Effective siRNA Delivery
3.1. Prediction of Enhanced Flexibility and siRNA Interactions of TEA-Core Dendrimers by Computer Simulations
3.2. High-Generation TEA-Core PAMAM Dendrimers as Effective In Vitro and In Vivo siRNA Nanocarriers
3.2.1. In Vitro Data
3.2.2. In Vivo Data
3.3. Low-Generation TEA-core PAMAM Dendrimers as Effective In Vitro and In Vivo siRNA Nanocarriers
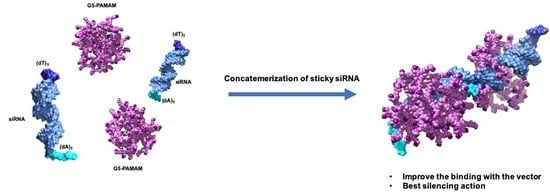
3.3.1. Functional Delivery of Sticky siRNA
3.3.2. In Vitro Preliminary Data of Sticky siRNA Delivery by Lower Generation TEA-Core PAMAMs
3.3.3. In Silico Binding Affinity of ssiRNAs with G5 TEA-Core Dendrimer Nanovectors
3.3.4. In Vitro Delivery of ssiRNAs with G5 TEA-Core Dendrimer Nanovectors
3.3.5. In Vivo Delivery of ssiRNAs with G5 TEA-Core Dendrimer Nanovectors
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Overhangs | ΔHbind | −TΔSbind | ΔGbind | Neff | ΔHbind,eff | −TΔSbind,eff | ΔGbind,eff |
|---|---|---|---|---|---|---|---|
| T2/T2 | −571.1 | 254.3 | −316.8 | 38 | −415.6 | 190.7 | −224.9 |
| T5/T5 | −609.9 | 265.1 | −344.8 | 41 | −503.1 | 241.1 | −262.0 |
| A5/A5 | −659.7 | 249.8 | −409.9 | 46 | −592.0 | 227.2 | −364.8 |
| A5/T5 | −637.4 | 250.0 | −387.4 | 44 | −554.9 | 233.4 | −321.5 |
| T7/T7 | −678.4 | 276.2 | −402.2 | 45 | −626.7 | 258.9 | −367.8 |
| A7/A7 | −714.8 | 266.9 | −447.9 | 52 | −669.1 | 243.6 | −425.5 |
| A7/T7 | −690.2 | 267.3 | −422.9 | 47 | −637.3 | 248.2 | −389.1 |
| Overhangs | ΔHbind | −TΔSbind | ΔGbind | ΔHbind,eff | −TΔSbind,eff | ΔGbind,eff | |
|---|---|---|---|---|---|---|---|
| (A5/A5)2 | −1382.7 | 407.3 | −975.4 | 96 | −1260.3 | 372.7 | −887.6 |
| (A7/T7)2 | −1480.4 | 455.9 | −1024.5 | 107 | −1441.2 | 437.2 | −1004.0 |
| (A5/T5)2 | 2 × (A5/T5) | (A7/T7)2 | 2 × (A7/T7) | |
|---|---|---|---|---|
| Neff | 96 | 88 | 107 | 94 |
| ΔHbind,eff | −1260.3 | −1109.8 | −1441.2 | −1274.6 |
| −TΔSbind,eff | 372.7 | 446.8 | 437.2 | 496.4 |
| ΔGbind,eff | −887.6 | −663.0 | −1004.0 | −778.2 |
References
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 2005, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Ameres, S.L.; Martinez, J.; Schroeder, R. Molecular basis for target RNA recognition and cleavage by RISC. Cell 2007, 131, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Bobbin, M.L.; Rossi, J.J. RNA interference (RNAi)-based therapeutics: Delivery on the promise? Annu. Rev. Pharmacol. Toxicol. 2016, 56, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Pecot, C.V.; Calin, G.A.; Coleman, R.L.; Lopez-Berestein, G.; Sood, A.K. RNA interference in the clinic: Challenges and future directions. Nat. Rev. Cancer 2011, 11, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Castanotto, D.; Rossi, J.J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 2009, 457, 426–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, I.A.; Yamada, Y.; Harashima, H. Optimization of siRNA delivery to target sites: Issues and future directions. Expert Opin. Drug Deliv. 2018, 15, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. Gene-silencing technology gets first drug approval after 20-year wait. Nature 2018, 560, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, C.; Gao, Y.; Li, X.; Tian, F.; Zhang, Y.; Fu, M.; Li, P.; Wang, Y.; Wang, F. Current transport systems and clinical applications for small interfering RNA (siRNA) drugs. Mol. Diagn. Ther. 2018, 22, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Durymanov, M.; Reineke, J. Non-viral delivery of nucleic acids: Insight into mechanisms of overcoming intracellular barriers. Front. Pharmacol. 2018, 9, 971. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Roy, R.; Shi, X.; Ceña, V.; El Kazzouli, S.; Majoral, J.P. Exploration of biomedical dendrimer space based on in-vivo physicochemical parameters: Key factor analysis. (Part 2). Drug Discov. Today 2019. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.V.; Santos, S.D.S.; Igne Ferreira, E.; Giarolla, J. New advances in general biomedical applications of PAMAM dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef] [PubMed]
- Leiro, V.; Santos, S.D.; Pego, A.P. Delivering siRNA with dendrimers: In vivo applications. Curr. Gene Ther. 2017, 17, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons and Dendritic Polymers: Discovery, Applications and the Future; Cambridge University Press: London, UK, 2012. [Google Scholar]
- Walter, M.V.; Malkoch, M. Simplifying the synthesis of dendrimers: Accelerated approaches. Chem. Soc. Rev. 2012, 41, 4593–4609. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, E.J.; Na, D.H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 2018, 41, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, H.; Liu, J.; Wang, Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm. 2018, 546, 215–225. [Google Scholar] [CrossRef]
- Luo, K.; He, B.; Wu, Y.; Shen, Y.; Gu, Z. Functional and biodegradable dendritic macromolecules with controlled architectures as nontoxic and efficient nanoscale gene vectors. Biotechnol. Adv. 2014, 32, 818–830. [Google Scholar] [CrossRef]
- Jędrych, M.; Borowska, K.; Galus, R.; Jodłowska-Jędrych, B. The evaluation of the biomedical effectiveness of poly(amido)amine dendrimers generation 4.0 as a drug and as drug carriers: A systematic review and meta-analysis. Int. J. Pharm. 2014, 462, 38–43. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.P. The proton sponge: A trick to enter cells viruses did not exploit. Chimia 1997, 51, 34–36. [Google Scholar]
- Haensler, J.; Szoka, F.C. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug. Chem. 1993, 4, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Kukowska-Latallo, J.F.; Bielinska, A.U.; Johnson, J.; Spindler, R.; Tomalia, D.A.; Baker, J.R., Jr. Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc. Natl. Acad. Sci. USA 1996, 93, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
- Eichman, J.D.; Bielinska, A.U.; Kukoswka-Latallo, J.F.; Baker, J.R., Jr. The use of PAMAM dendrimers in the efficient transfer of generic material into cells. Pharm. Sci. Technol. Today 2000, 3, 232–245. [Google Scholar] [CrossRef]
- Guillot-Nieckowski, M.; Eisler, S.; Diederich, F. Dendritic vectors for gene transfection. New J. Chem. 2007, 31, 1111–1127. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Non viral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, X.; Peng, L. Molecular engineering of dendrimer nanovectors for siRNA delivery and gene silencing. Front. Chem. Sci. Eng. 2017, 11, 663–675. [Google Scholar] [CrossRef]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Kesharwani, P.; Benerjee, S.; Gupta, U.; Amin, M.C.I.M.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. PAMAM dendrimers as promising nanocarriers for RNAi therapeutics. Mater. Today 2015, 18, 565–572. [Google Scholar] [CrossRef]
- Liu, X.; Rocchi, P.; Peng, L. Dendrimers as non-viral vectors for siRNA delivery. New J. Chem. 2012, 36, 256–263. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, J.; Hafdi, N.; Behr, J.P.; Erbacher, P.; Peng, L. PAMAM dendrimers for efficient siRNA delivery and potent gene silencing. Chem. Commun. 2006, 22, 2362–2364. [Google Scholar] [CrossRef]
- Venkatesh, S.; Workman, J.L. Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell. Biol. 2015, 16, 178–189. [Google Scholar] [CrossRef]
- Karatasos, K.; Posocco, P.; Laurini, E.; Pricl, S. Poly(amidoamine)-based dendrimer/siRNA complexation studied by computer simulations: Effects of pH and generation on dendrimer structure and siRNA binding. Macromol. Biosci. 2012, 12, 225–240. [Google Scholar] [CrossRef]
- Posocco, P.; Laurini, E.; Dal Col, V.; Marson, D.; Karatasos, K.; Fermeglia, M.; Pricl, S. Tell me something that I do not know. Multiscale molecular modeling of dendrimer/dendron organization and self-assembly in gene therapy. Curr. Med. Chem. 2012, 19, 5062–5087. [Google Scholar] [CrossRef]
- Posocco, P.; Laurini, E.; Dal Col, V.; Marson, D.; Peng, L.; Smith, D.K.; Klajnert, B.; Bryszewska, M.; Caminade, A.-M.; Majoral, J.P.; et al. Multiscale modeling of dendrimers and dendrons for drug and nucleic acid delivery. In Dendrimers in Biomedical Applications; Klajnert, B., Peng, L., Ceña, V., Eds.; RSC Publishing: Cambrige, UK, 2013; pp. 148–166. [Google Scholar]
- Pavan, G.M.; Posocco, P.; Tagliabue, A.; Maly, M.; Malek, A.; Danani, A.; Ragg, E.; Catapano, C.V.; Pricl, S. PAMAM dendrimers for siRNA delivery: Computational and experimental insights. Chem. Eur. J. 2010, 16, 7781–7795. [Google Scholar] [CrossRef]
- Marson, D.; Laurini, E.; Posocco, P.; Fermeglia, M.; Pricl, S. Cationic carbosilane dendrimers and oligonucleotide binding: An energetic affair. Nanoscale 2015, 7, 3876–3887. [Google Scholar] [CrossRef]
- Mehrabadi, F.S.; Hirsch, O.; Zeisig, R.; Posocco, P.; Laurini, E.; Pricl, S.; Haag, R.; Kemmner, W.; Calderón, M. Structure–activity relationship study of dendritic polyglycerolamines for efficient siRNA transfection. RSC Adv. 2015, 5, 78760–78770. [Google Scholar] [CrossRef]
- Shen, X.-C.; Zhou, J.; Liu, X.; Wu, J.; Qu, F.; Zhang, Z.-L.; Pang, D.-W.; Quélèver, G.; Zhang, C.-C.; Peng, L. Importance of size-to-charge ratio in construction of stable and uniform nanoscale RNA/dendrimer complexes. Org. Biomol. Chem. 2007, 5, 3674–3681. [Google Scholar] [CrossRef]
- Liu, X.-X.; Rocchi, P.; Qu, F.; Zheng, S.-Q.; Liang, Z.; Gleave, M.; Iovanna, J.; Peng, L. PAMAM dendrimers mediate siRNA delivery to target Hsp27 and produce potent antiproliferative effects on prostate cancer cells. ChemMedChem 2009, 4, 1302–1310. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, X.; Liang, X.; Jiang, G. Molecular and cellular mechanisms of castration resistant prostate cancer. Oncol. Lett. 2018, 15, 6063–6076. [Google Scholar] [CrossRef]
- Rocchi, P.; So, A.; Kojima, S.; Signaevsky, M.; Beraldi, E.; Fazli, L.; Hurtado-Coll, A.; Yamanaka, K.; Gleave, M. Heat shock protein 27 increases after androgen ablation and plays a cytoprotective role in hormone-refractory prostate cancer. Cancer Res. 2004, 64, 6595–6602. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Catapano, C.V.; Peng, L.; Zhou, J.; Rocchi, P. Structurally flexible triethanolamine-core dendrimers as effective nanovectors to deliver RNAi-based therapeutics. Biotechnol. Adv. 2014, 32, 844–852. [Google Scholar] [CrossRef]
- Kala, S.; Mak, A.S.C.; Liu, X.; Posocco, P.; Pricl, S.; Peng, L.; Wong, A.S.T. Combination of dendrimer-nanovector-mediated small interfering RNA delivery to target AKT with the clinical anticancer drug paclitaxel for effective and potent anticancer activity in treating ovarian cancer. J. Med. Chem. 2014, 57, 2634–2642. [Google Scholar] [CrossRef]
- Reebye, V.; Sætrom, P.; Mintz, P.J.; Huang, K.W.; Swiderski, P.; Peng, L.; Liu, C.; Liu, X.; Lindkaer-Jensen, S.; Zacharoulis, D.; et al. Novel RNA oligonucleotide improves liver function and inhibits liver carcinogenesis in vivo. Hepatology 2014, 59, 216–227. [Google Scholar] [CrossRef]
- Cui, Q.; Yang, S.; Ye, P.; Tian, E.; Sun, G.; Zhou, J.; Sun, G.; Liu, X.; Chen, C.; Murai, K.; et al. Downregulation of TLX induces TET3 expression and inhibits glioblastoma stem cell self-renewal and tumorigenesis. Nat. Commun. 2016, 7, 10637–10651. [Google Scholar] [CrossRef]
- Lang, M.F.; Yang, S.; Zhao, C.; Sun, G.; Murai, K.; Wu, X.; Wang, J.; Gao, H.; Brown, C.E.; Liu, X.; et al. Genome-wide profiling identified a set of miRNAs that are differentially expressed in glioblastoma stem cells and normal neural stem cells. PLoS ONE 2012, 7, e36248–e36251. [Google Scholar] [CrossRef]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.H. AKT as a therapeutic target for cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef]
- Christie, E.L.; Bowtell, D.D.L. Acquired chemotherapy resistance in ovarian cancer. Ann. Oncol. 2017, 28 (Suppl. 8), viii13–viii15. [Google Scholar] [CrossRef]
- Svenson, S. The dendrimer paradox—Highly medical expectations but poor clinical translation. Chem. Soc. Rev. 2015, 44, 2228–2238. [Google Scholar] [CrossRef]
- Bolcato-Bellemin, A.L.; Bonnet, M.E.; Creusat, G.; Erbacher, P.; Behr, J.P. Sticky overhangs enhance siRNA-mediated gene silencing. Proc. Natl. Acad. Sci. USA 2007, 104, 16050–16055. [Google Scholar] [CrossRef] [Green Version]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethyleneimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Laurini, E.; Posocco, P.; Pricl, S.; Qu, F.; Rocchi, P.; Peng, L. Efficient delivery of sticky siRNA and potent gene silencing in a prostate cancer model using a generation 5 triethanolamine-core PAMAM dendrimer. Mol. Pharm. 2012, 9, 470–481. [Google Scholar] [CrossRef]
- Posocco, P.; Liu, X.; Laurini, E.; Marson, D.; Chen, C.; Liu, C.; Fermeglia, M.; Rocchi, P.; Pricl, S.; Peng, L. Impact of siRNA overhang for dendrimer-mediated siRNA delivery and gene silencing. Mol. Pharm. 2013, 10, 3262–3273. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- First-in-Human Safety and Tolerability Study of MTL-CEBPA in Patients with Advanced Liver Cancer (OUTREACH). Available online: https://clinicaltrials.gov/ct2/show/NCT02716012 (accessed on 17 July 2019).



















| TEA-Core PAMAMs | NH3-Core PAMAMs | |||||
|---|---|---|---|---|---|---|
| G | ΔGbind/N | ΔHbind/N | −TΔSbind/N | ΔGbind/N | ΔHbind/N | −TΔSbind/N |
| 4 | −7.57 1 | −9.82 | 2.25 | −4.57 | −8.02 | 3.45 |
| 5 | −14.9 | −17.9 | 3.02 | −11.5 | −16.0 | 4.43 |
| 6 | −17.0 | −20.5 | 3.55 | −14.1 | −18.8 | 4.77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marson, D.; Laurini, E.; Aulic, S.; Fermeglia, M.; Pricl, S. Evolution from Covalent to Self-Assembled PAMAM-Based Dendrimers as Nanovectors for siRNA Delivery in Cancer by Coupled In Silico-Experimental Studies. Part I: Covalent siRNA Nanocarriers. Pharmaceutics 2019, 11, 351. https://doi.org/10.3390/pharmaceutics11070351
Marson D, Laurini E, Aulic S, Fermeglia M, Pricl S. Evolution from Covalent to Self-Assembled PAMAM-Based Dendrimers as Nanovectors for siRNA Delivery in Cancer by Coupled In Silico-Experimental Studies. Part I: Covalent siRNA Nanocarriers. Pharmaceutics. 2019; 11(7):351. https://doi.org/10.3390/pharmaceutics11070351
Chicago/Turabian StyleMarson, Domenico, Erik Laurini, Suzana Aulic, Maurizio Fermeglia, and Sabrina Pricl. 2019. "Evolution from Covalent to Self-Assembled PAMAM-Based Dendrimers as Nanovectors for siRNA Delivery in Cancer by Coupled In Silico-Experimental Studies. Part I: Covalent siRNA Nanocarriers" Pharmaceutics 11, no. 7: 351. https://doi.org/10.3390/pharmaceutics11070351
APA StyleMarson, D., Laurini, E., Aulic, S., Fermeglia, M., & Pricl, S. (2019). Evolution from Covalent to Self-Assembled PAMAM-Based Dendrimers as Nanovectors for siRNA Delivery in Cancer by Coupled In Silico-Experimental Studies. Part I: Covalent siRNA Nanocarriers. Pharmaceutics, 11(7), 351. https://doi.org/10.3390/pharmaceutics11070351