Pharmacokinetics of Intravitreal Anti-VEGF Drugs in Age-Related Macular Degeneration
Abstract
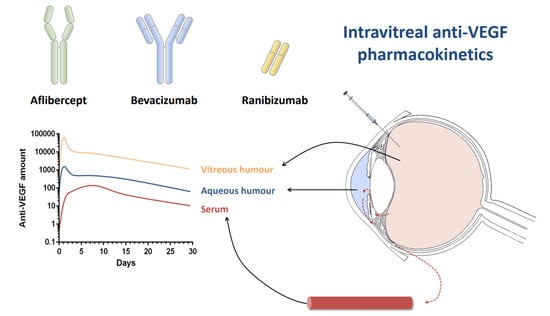
:1. Introduction
2. Pharmacokinetics of Anti-VEFG Drugs
2.1. Ranibizumab
2.1.1. Animal Studies
2.1.2. Human Studies
2.2. Bevacizumab
2.2.1. Animal Studies
2.2.2. Human Studies
2.3. Aflibercept
2.3.1. Animal Studies
2.3.2. Human Studies
3. Pharmacokinetic Considerations
3.1. Eye Physiological Factors
3.1.1. Distribution-Diffusion in the Vitreous Humour
3.1.2. Elimination of Drugs from the Vitreous Humour
3.2. Surgical Ocular Procedures (Lensectomy and Vitrectomy)
3.3. Analytical Methods Used in Pharmacokinetic Studies
4. Outlooks
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Ferris, F.L.; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Beckman Initiative for Macular Research Classification Committee Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- De Jong, P.T.V.M. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, B.; Frazer-Abel, A.; Leonard, A.; Ratnapriya, R.; Ward, T.; Pietraszkiewicz, A.; O’Quinn, E.; Adams, K.; Swaroop, A.; Wolf, B.J. Association of age-related macular degeneration with complement activation products, smoking, and single nucleotide polymorphisms in South Carolinians of European and African descent. Mol. Vis. 2019, 25, 79–92. [Google Scholar] [PubMed]
- Empeslidis, T.; Storey, M.; Giannopoulos, T.; Konidaris, V.; Tranos, P.G.; Panagiotou, E.S.; Voudouragkaki, I.C.; Konstas, A.G. How Successful is Switching from Bevacizumab or Ranibizumab to Aflibercept in Age-Related Macular Degeneration? A Systematic Overview. Adv. Ther. 2019, 36, 1532–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pershing, S.; Talwar, N.; Armenti, S.T.; Grubbs, J.; Rosenthal, J.M.; Dedania, V.S.; Stein, J.D. Use of Bevacizumab and Ranibizumab for Wet Age-Related Macular Degeneration: Influence of CATT Results and Introduction of Aflibercept. Am. J. Ophthalmol. 2019. [Google Scholar] [CrossRef]
- Cioffi, C.L.; Johnson, G.; Petrukhin, K. Recent Developments in Agents for the Treatment of Age-related Macular Degeneration and Stargardt Disease. In 2016 Medicinal Chemistry Reviews; Desai, M.C., Ed.; MEDI Inc.: Baltimore, MA, USA, 2016; Volume 51, pp. 261–281. ISBN 978-0-9962932-3-5. [Google Scholar]
- Neumann, R.; Barequet, D. The gap between the need for novel retinal drug delivery methods, technologies in R&D phase, and approved ocular drug delivery technologies. Drug Discov. Today 2019. [Google Scholar] [CrossRef]
- Brown, D.M.; Michels, M.; Kaiser, P.K.; Heier, J.S.; Sy, J.P.; Ianchulev, T. ANCHOR Study Group Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology 2009, 116, 57–65.e5. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.-F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). OPH 2017, 237, 185–222. [Google Scholar] [CrossRef] [PubMed]
- García-Layana, A.; Figueroa, M.S.; Arias, L.; Araiz, J.; Ruiz-Moreno, J.M.; García-Arumí, J.; Gómez-Ulla, F.; López-Gálvez, M.I.; Cabrera-López, F.; García-Campos, J.M.; et al. Individualized Therapy with Ranibizumab in Wet Age-Related Macular Degeneration. J. Ophthalmol. 2015, 2015, 412903. [Google Scholar] [CrossRef] [PubMed]
- Cost comparison table of anti-VEGF therapies for W-AMD. In Aflibercept (Eylea): Treatment of Neovascular (Wet) Age-Related Macular Degeneration (wAMD); CADTH Common Drug Reviews; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2015.
- Okada, M.; Kandasamy, R.; Chong, E.W.; McGuiness, M.; Guymer, R.H. The Treat-and-Extend Injection Regimen Versus Alternate Dosing Strategies in Age-related Macular Degeneration: A Systematic Review and Meta-analysis. Am. J. Ophthalmol. 2018, 192, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Haga, A.; Kawaji, T.; Ideta, R.; Inomata, Y.; Tanihara, H. Treat-and-extend versus every-other-month regimens with aflibercept in age-related macular degeneration. Acta Ophthalmol. 2018, 96, e393–e398. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Le, K.; et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 2014, 98, 1636–1641. [Google Scholar] [CrossRef]
- Bhagat, R.; Zhang, J.; Farooq, S.; Li, X.-Y. Comparison of the release profile and pharmacokinetics of intact and fragmented dexamethasone intravitreal implants in rabbit eyes. J. Ocul. Pharmacol. Ther. 2014, 30, 854–858. [Google Scholar] [CrossRef]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Ezzat, M.K.; Singh, R.J. Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 2007, 114, 2179–2182. [Google Scholar] [CrossRef]
- Xu, L.; Lu, T.; Tuomi, L.; Jumbe, N.; Lu, J.; Eppler, S.; Kuebler, P.; Damico-Beyer, L.A.; Joshi, A. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: A population approach. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1616–1624. [Google Scholar] [CrossRef]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.K.; Liddell, M.R.; Wen, H. Effective electrophoretic mobilities and charges of anti-VEGF proteins determined by capillary zone electrophoresis. J. Pharm. Biomed. Anal. 2011, 55, 603–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holash, J.; Davis, S.; Papadopoulos, N.; Croll, S.D.; Ho, L.; Russell, M.; Boland, P.; Leidich, R.; Hylton, D.; Burova, E.; et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. USA 2002, 99, 11393–11398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christoforidis, J.B.; Carlton, M.M.; Knopp, M.V.; Hinkle, G.H. PET/CT imaging of I-124-radiolabeled bevacizumab and ranibizumab after intravitreal injection in a rabbit model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5899–5903. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Ahn, J.; Park, S.; Kim, H.; Hwang, D.J.; Park, J.H.; Park, J.Y.; Chung, J.Y.; Park, K.H.; Woo, S.J. Intraocular pharmacokinetics of ranibizumab in vitrectomized versus nonvitrectomized eyes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, J.; Fei, D.; Beyer, J.C.; Ryan, A.; Rangell, L.; Shiu, V.; Damico, L.A. Pharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbits. Retina 2007, 27, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, J.B.; Briley, K.; Binzel, K.; Bhatia, P.; Wei, L.; Kumar, K.; Knopp, M.V. Systemic Biodistribution and Intravitreal Pharmacokinetic Properties of Bevacizumab, Ranibizumab, and Aflibercept in a Nonhuman Primate Model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5636–5645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudreault, J.; Fei, D.; Rusit, J.; Suboc, P.; Shiu, V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Investig. Ophthalmol. Vis. Sci. 2005, 46, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Maia, M.; et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 2017, 37, 1847–1858. [Google Scholar] [CrossRef]
- Krohne, T.U.; Liu, Z.; Holz, F.G.; Meyer, C.H. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am. J. Ophthalmol. 2012, 154, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.W. What are the half-lives of ranibizumab and aflibercept (VEGF Trap-eye) in human eyes? Calculations with a mathematical model. Eye Rep. 2011, 1, 5. [Google Scholar] [CrossRef]
- Moisseiev, E.; Waisbourd, M.; Ben-Artsi, E.; Levinger, E.; Barak, A.; Daniels, T.; Csaky, K.; Loewenstein, A.; Barequet, I.S. Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Tew, W.P.; Gordon, M.; Murren, J.; Dupont, J.; Pezzulli, S.; Aghajanian, C.; Sabbatini, P.; Mendelson, D.; Schwartz, L.; Gettinger, S.; et al. Phase 1 study of aflibercept administered subcutaneously to patients with advanced solid tumors. Clin. Cancer Res. 2010, 16, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sawada, T.; Sawada, O.; Saishin, Y.; Liu, P.; Ohji, M. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Zehetner, C.; Kirchmair, R.; Huber, S.; Kralinger, M.T.; Kieselbach, G.F. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br. J. Ophthalmol 2013, 97, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Zehetner, C.; Kralinger, M.T.; Modi, Y.S.; Waltl, I.; Ulmer, H.; Kirchmair, R.; Bechrakis, N.E.; Kieselbach, G.F. Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: A randomised, prospective trial. Acta Ophthalmol. 2015, 93, e154–e159. [Google Scholar] [CrossRef] [PubMed]
- Reimbursement by a National Healthcare Insurance System of a Medicinal Product for a Use Not Covered by Its Marketing Authorisation (Off-Label Use). Available online: http://curia.europa.eu/juris/document/document.jsf?text=&docid=207947&pageIndex=0&doclang=en&mode=req&dir=&occ=first&part=1 (accessed on 29 May 2019).
- Dakin, H.A.; Wordsworth, S.; Rogers, C.A.; Abangma, G.; Raftery, J.; Harding, S.P.; Lotery, A.J.; Downes, S.M.; Chakravarthy, U.; Reeves, B.C.; et al. Cost-effectiveness of ranibizumab and bevacizumab for age-related macular degeneration: 2-year findings from the IVAN randomised trial. BMJ Open 2014, 4, e005094. [Google Scholar] [CrossRef] [PubMed]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Singh, R.J. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007, 114, 855–859. [Google Scholar] [CrossRef]
- Nomoto, H.; Shiraga, F.; Kuno, N.; Kimura, E.; Fujii, S.; Shinomiya, K.; Nugent, A.K.; Hirooka, K.; Baba, T. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4807–4813. [Google Scholar] [CrossRef]
- Sinapis, C.I.; Routsias, J.G.; Sinapis, A.I.; Sinapis, D.I.; Agrogiannis, G.D.; Pantopoulou, A.; Theocharis, S.E.; Baltatzis, S.; Patsouris, E.; Perrea, D. Pharmacokinetics of intravitreal bevacizumab (Avastin®) in rabbits. Clin. Ophthalmol 2011, 5, 697–704. [Google Scholar] [CrossRef]
- Miyake, T.; Sawada, O.; Kakinoki, M.; Sawada, T.; Kawamura, H.; Ogasawara, K.; Ohji, M. Pharmacokinetics of bevacizumab and its effect on vascular endothelial growth factor after intravitreal injection of bevacizumab in macaque eyes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1606–1608. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, J.B.; Williams, M.M.; Kothandaraman, S.; Kumar, K.; Epitropoulos, F.J.; Knopp, M.V. Pharmacokinetic properties of intravitreal I-124-aflibercept in a rabbit model using PET/CT. Curr. Eye Res. 2012, 37, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Dınc, E.; Yıldırım, O.; Necat Yılmaz, S.; Canacankatan, N.; Ayaz, L.; Ozcan, T.; Temel, G.O. Intravitreal bevacizumab effects on VEGF levels in distant organs: An experimental study. Cutan. Ocul. Toxicol. 2014, 33, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Ziemssen, F.; Henke-Fahle, S.; Tatar, O.; Szurman, P.; Aisenbrey, S.; Schneiderhan-Marra, N.; Xu, X. Tübingen Bevacizumab Study Group; Grisanti, S. Vitreous levels of bevacizumab and vascular endothelial growth factor-A in patients with choroidal neovascularization. Ophthalmology 2008, 115, 1750–1755. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liang, X.H.; Ferrara, N. Comparing protein VEGF inhibitors: In vitro biological studies. Biochem. Biophys. Res. Commun. 2011, 408, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Dedania, V.S.; Bakri, S.J. Systemic safety of intravitreal anti-vascular endothelial growth factor agents in age-related macular degeneration. Curr. Opin. Ophthalmol. 2016, 27, 224–243. [Google Scholar] [CrossRef] [PubMed]
- Moja, L.; Lucenteforte, E.; Kwag, K.H.; Bertele, V.; Campomori, A.; Chakravarthy, U.; D’Amico, R.; Dickersin, K.; Kodjikian, L.; Lindsley, K.; et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2014, CD011230. [Google Scholar] [CrossRef]
- Thulliez, M.; Angoulvant, D.; Le Lez, M.L.; Jonville-Bera, A.-P.; Pisella, P.-J.; Gueyffier, F.; Bejan-Angoulvant, T. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: Systematic review and meta-analysis. JAMA Ophthalmol. 2014, 132, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Krohne, T.U.; Eter, N.; Holz, F.G.; Meyer, C.H. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am. J. Ophthalmol. 2008, 146, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.H.; Krohne, T.U.; Holz, F.G. Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg versus 3.0 mg of bevacizumab in humans. Retina 2011, 31, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.H.; Krohne, T.U.; Holz, F.G. Concentrations of unbound bevacizumab in the aqueous of untreated fellow eyes after a single intravitreal injection in humans. Acta Ophthalmol. 2012, 90, 68–70. [Google Scholar] [CrossRef]
- Park, S.J.; Choi, Y.; Na, Y.M.; Hong, H.K.; Park, J.Y.; Park, K.H.; Chung, J.Y.; Woo, S.J. Intraocular Pharmacokinetics of Intravitreal Aflibercept (Eylea) in a Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
- Niwa, Y.; Kakinoki, M.; Sawada, T.; Wang, X.; Ohji, M. Ranibizumab and Aflibercept: Intraocular Pharmacokinetics and Their Effects on Aqueous VEGF Level in Vitrectomized and Nonvitrectomized Macaque Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6501–6505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, P.K.; Kodjikian, L.; Korobelnik, J.-F.; Winkler, J.; Torri, A.; Zeitz, O.; Vitti, R.; Ahlers, C.; Zimmermann, T.; Dicioccio, A.T.; et al. Systemic pharmacokinetic/pharmacodynamic analysis of intravitreal aflibercept injection in patients with retinal diseases. BMJ. Open Ophthalmol. 2019, 4, e000185. [Google Scholar] [CrossRef]
- Stewart, M.W. Pharmacokinetics, pharmacodynamics and pre-clinical characteristics of ophthalmic drugs that bind VEGF. Expert Rev. Clin. Pharmacol 2014, 7, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Do, D.V.; Rhoades, W.; Nguyen, Q.D. Pharmacokinetic study of intravitreal aflibercept in humans with neovascular age-related macular degeneration. Retina 2019. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, J.B.; Williams, M.M.; Wang, J.; Jiang, A.; Pratt, C.; Abdel-Rasoul, M.; Hinkle, G.H.; Knopp, M.V. Anatomic and pharmacokinetic properties of intravitreal bevacizumab and ranibizumab after vitrectomy and lensectomy. Retina 2013, 33, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Christoforidis, J.B.; Xie, Z.; Jiang, A.; Wang, J.; Pratt, C.; Gemensky-Metzler, A.; Abdel-Rasoul, M.; Roy, S.; Liu, Z. Serum levels of intravitreal bevacizumab after vitrectomy, lensectomy and non-surgical controls. Curr. Eye Res. 2013, 38, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kim, H.; Woo, S.J.; Park, J.H.; Park, S.; Hwang, D.J.; Park, K.H. Pharmacokinetics of intravitreally injected bevacizumab in vitrectomized eyes. J. Ocul. Pharmacol. Ther. 2013, 29, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, J.; Zhang, Y.; Liu, Q.; Bai, R.; Yuan, W.; Cai, D.; Zheng, X.; Bian, Y.; Zhou, S.; et al. Ocular Biodistribution of 89Zr-Bevacizumab in New Zealand Rabbits Determined Using PET/MRI: A Feasibility Study. Iran. J. Radiol. 2019, 16. [Google Scholar] [CrossRef]
- Kakinoki, M.; Sawada, O.; Sawada, T.; Saishin, Y.; Kawamura, H.; Ohji, M. Effect of vitrectomy on aqueous VEGF concentration and pharmacokinetics of bevacizumab in macaque monkeys. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5877–5880. [Google Scholar] [CrossRef] [PubMed]
- Missel, P.J. Simulating intravitreal injections in anatomically accurate models for rabbit, monkey, and human eyes. Pharm. Res. 2012, 29, 3251–3272. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Boylan, N.J.; Suk, J.S.; Wang, Y.-Y.; Nance, E.A.; Yang, J.-C.; McDonnell, P.J.; Cone, R.A.; Duh, E.J.; Hanes, J. Nanoparticle diffusion in, and microrheology of, the bovine vitreous ex vivo. J. Control Release 2013, 167, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laude, A.; Tan, L.E.; Wilson, C.G.; Lascaratos, G.; Elashry, M.; Aslam, T.; Patton, N.; Dhillon, B. Intravitreal therapy for neovascular age-related macular degeneration and inter-individual variations in vitreous pharmacokinetics. Prog. Retin. Eye Res. 2010, 29, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Angi, M.; Kalirai, H.; Coupland, S.E.; Damato, B.E.; Semeraro, F.; Romano, M.R. Proteomic analyses of the vitreous humour. Mediat. Inflamm. 2012, 2012, 148039. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.N.; Spannagl, M.; Kampik, A.; Gandorfer, A. Components of the fibrinolytic system in the vitreous body in patients with vitreoretinal disorders. Clin. Experiment. Ophthalmol. 2008, 36, 431–436. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Gal-Or, O.; Dotan, A.; Dachbash, M.; Tal, K.; Nisgav, Y.; Weinberger, D.; Ehrlich, R.; Livnat, T. Bevacizumab clearance through the iridocorneal angle following intravitreal injection in a rat model. Exp. Eye Res. 2016, 145, 412–416. [Google Scholar] [CrossRef]
- Peters, S.; Heiduschka, P.; Julien, S.; Ziemssen, F.; Fietz, H.; Bartz-Schmidt, K.U. Tübingen Bevacizumab Study Group; Schraermeyer, U. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am. J. Ophthalmol. 2007, 143, 995–1002. [Google Scholar] [CrossRef]
- Heiduschka, P.; Fietz, H.; Hofmeister, S.; Schultheiss, S.; Mack, A.F.; Peters, S.; Ziemssen, F.; Niggemann, B.; Julien, S.; Bartz-Schmidt, K.U.; et al. Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2814–2823. [Google Scholar] [CrossRef]
- Vellonen, K.-S.; Hellinen, L.; Mannermaa, E.; Ruponen, M.; Urtti, A.; Kidron, H. Expression, activity and pharmacokinetic impact of ocular transporters. Adv. Drug Deliv. Rev. 2018, 126, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Krohne, T.U.; Muether, P.S.; Stratmann, N.K.; Holz, F.G.; Kirchhof, B.; Meyer, C.H.; Fauser, S. Influence of ocular volume and lens status on pharmacokinetics and duration of action of intravitreal vascular endothelial growth factor inhibitors. Retina 2015, 35, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Edington, M.; Connolly, J.; Chong, N.V. Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes. Expert Opin Drug Metab Toxicol 2017, 13, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Gisladottir, S.; Loftsson, T.; Stefansson, E. Diffusion characteristics of vitreous humour and saline solution follow the Stokes Einstein equation. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Stefánsson, E. Physiology of vitreous surgery. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; You, Y.; Du, W.; Zhao, C.; Li, J.; Mao, J.; Chen, H.; Cheng, L. Ocular pharmacokinetics of bevacizumab in vitrectomized eyes with silicone oil tamponade. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5221–5226. [Google Scholar] [CrossRef] [PubMed]
- Gadkar, K.; Pastuskovas, C.V.; Le Couter, J.E.; Elliott, J.M.; Zhang, J.; Lee, C.V.; Sanowar, S.; Fuh, G.; Kim, H.S.; Lombana, T.N.; et al. Design and Pharmacokinetic Characterization of Novel Antibody Formats for Ocular Therapeutics. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5390–5400. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Maia, M.; Wakshull, E.; Siguenza, P.; Liu, P.; Lakhani, S.; Rusit, J.; Elliott, R.; Quarmby, V. Development of a novel homogenous electrochemiluminescence assay for quantitation of ranibizumab in human serum. J. Pharm. Biomed. Anal. 2010, 52, 680–686. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, Z.; Kaila, N.; Kuebler, P.; Visich, J.; Maia, M.; Tuomi, L.; Ehrlich, J.S.; Rubio, R.G.; Campochiaro, P.A. Pharmacokinetics of ranibizumab after intravitreal administration in patients with retinal vein occlusion or diabetic macular edema. Ophthalmology 2014, 121, 2237–2246. [Google Scholar] [CrossRef]
- Lowe, J.; Wakshull, E.; Shek, T.; Chuntharapai, A.; Elliott, R.; Rusit, J.; Maia, M. Development and validation of a novel semi-homogenous clinical assay for quantitation of Ranibizumab in human serum. J. Immunol. Methods 2018, 461, 44–52. [Google Scholar] [CrossRef]
- Dickmann, L.J.; Yip, V.; Li, C.; Abundes, J.; Maia, M.; Young, C.; Stainton, S.; Hass, P.E.; Joseph, S.B.; Prabhu, S.; et al. Evaluation of Fluorophotometry to Assess the Vitreal Pharmacokinetics of Protein Therapeutics. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6991–6999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannos, S.A.; Kraft, E.R.; Zhao, Z.-Y.; Merkley, K.H.; Cai, J. Formulation Stabilization and Disaggregation of Bevacizumab, Ranibizumab and Aflibercept in Dilute Solutions. Pharm. Res. 2018, 35, 78. [Google Scholar] [CrossRef] [PubMed]
- Muether, P.S.; Hermann, M.M.; Dröge, K.; Kirchhof, B.; Fauser, S. Long-term stability of vascular endothelial growth factor suppression time under ranibizumab treatment in age-related macular degeneration. Am. J. Ophthalmol. 2013, 156, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.J.; Muether, P.S.; Fauser, S. A model of the ocular pharmacokinetics involved in the therapy of neovascular age-related macular degeneration with ranibizumab. Br. J. Ophthalmol. 2015, 99, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Celik, N.; Scheuerle, A.; Auffarth, G.U.; Kopitz, J.; Dithmar, S. Intraocular Pharmacokinetics of Aflibercept and Vascular Endothelial Growth Factor-A. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5574–5578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mould, D.R.; Upton, R.N. Basic concepts in population modeling, simulation, and model-based drug development. CPT Pharmacomet. Syst Pharmacol 2012, 1, e6. [Google Scholar] [CrossRef] [PubMed]
- Sheiner, L.B.; Rosenberg, B.; Melmon, K.L. Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput. Biomed. Res. 1972, 5, 411–459. [Google Scholar] [CrossRef]
- Sheiner, L.B.; Beal, S.L. Evaluation of methods for estimating population pharmacokinetics parameters. I. Michaelis-Menten model: Routine clinical pharmacokinetic data. J. Pharmacokinet. Biopharm. 1980, 8, 553–571. [Google Scholar] [CrossRef] [PubMed]
- Stanski, D.R.; Maitre, P.O. Population pharmacokinetics and pharmacodynamics of thiopental: The effect of age revisited. Anesthesiology 1990, 72, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Manolis, E.; Brogren, J.; Cole, S.; Hay, J.L.; Nordmark, A.; Karlsson, K.E.; Lentz, F.; Benda, N.; Wangorsch, G.; Pons, G.; et al. Commentary on the MID3 Good Practices Paper. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 416–417. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Burghaus, R.; Cosson, V.; Cheung, S.; Chenel, M.; DellaPasqua, O.; Frey, N.; Hamrén, B.; Harnisch, L.; Ivanow, F.; et al. Good Practices in Model-Informed Drug Discovery and Development: Practice, Application, and Documentation. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 93–122. [Google Scholar]

| Properties |  |  |  |
|---|---|---|---|
| Ranibizumab | Bevacizumab | Aflibercept | |
| Class | Antibody fragment | Monoclonal antibody | Fusion protein |
| MW (KDa) | 48 | 149 | 115 |
| Net charge | Negative | Negative | Slightly positive |
| Binding target | VEGF-A | VEGF-A | VEGF-A, VEGF-B, PIGF |
| KD for VEGF165 (pM) | 46 | 58 | 0.49 |
| Model | Injected Dose | Determination | Sensitivity | PK Model | Sample | Time Points | Normal Eyes | Vitrectomy | Aphakia | Observations | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t1/2 | Tmax | Cmax | AUC | t1/2 | Cmax | AUC | t1/2 | |||||||||
| Dutch-belted rabbits | 0.5 mg/0.05 mL | CLIA | LLOQ = 0.375 ng/mL | NC | VH | 1, 3, 8, 15, 29 days | 2.88 days | 1 day | 162 µg/mL | No detection in serum | [21] | |||||
| AH | 2.84 days | 3 days | 17.9 µg/mL | |||||||||||||
| Serum | ||||||||||||||||
| Dutch-belted rabbits | 0.5 mg/0.05 mL | PET (I-124) | 2C | VH | 0, 2, 5, 7, 14, 21, 28, 35 days | 2.81 days | 0 h | No detection in other organs | [26] | |||||||
| AH | ||||||||||||||||
| Serum | ||||||||||||||||
| Dutch-belted rabbits | 0.5 mg/0.05 mL | PET (I-124) | 2C | VH | 0, 2, 5, 7, 14, 21, 28, 35 days | 2.81 days | 2.13 days | 1.79 days | [60] | |||||||
| AH | ||||||||||||||||
| Serum | ||||||||||||||||
| New Zealand rabbits | 0.625 mg/0.05 mL | ELISA | 0.78 ng/mL | 1C | VH | 1, 8 h; 1, 2, 4, 7, 14, 21, 30, 42, 50, 60 days | 2.9 days | 1 h | 1280 µg/mL | Bilateral injection | [28] | |||||
| 0.78 ng/mL | NC | AH | 3 days | 48 h | 57.1 µg/mL | |||||||||||
| 7.8 ng/mL | NC | Serum | 24 h | 0.055 µg/mL | ||||||||||||
| New Zealand rabbits | 0.25 mg/0.025 mL | ELISA | LLOQ = 0.375 ng/mL | 1C | VH | 1 h or 1, 2, 5, 14, 30 days | 2.75 days | 1 h | 91.61 µg/mL | 2.51 days | 118.01 µg/mL | [27] | ||||
| AH | 1 h | 20.38 µg/mL | 21.7 µg/mL | |||||||||||||
| Serum | ||||||||||||||||
| Owl monkeys | 0.5 mg/0.05 mL | PET (I-124) | 2C | VH | 0, 1, 2, 4, 8, 14, 21, 28, 35 days | 2.73 days | [29] | |||||||||
| AH | ||||||||||||||||
| Serum | 1, 2, 4, 8, 12 h; 1, 2, 4, 8, 14, 21, 28, 35 days | 24 h | 0.47 ng/mL | |||||||||||||
| Cynomolgus macaques | 0.5 mg/0.05 mL | ELISA | 1.5 ng/mL | 1C | VH | 6 h, 2, 3, 5, 8, 11 days | 2.63 days | 6 h | 169 µg/mL | Bilateral injection | [30] | |||||
| 1.5 ng/mL | NC | AH | 2.54 days | 6 h | 116 µg/mL | |||||||||||
| 15.6 ng/mL | NC | Serum | 2, 6, 12, 24, 36, 48 h; 4–11 days | 3.59 days | 6 h | 150 µg/mL | ||||||||||
| Cynomolgus macaques | 2 mg/0.05 mL | ELISA | 1.5 ng/mL | 1C | VH | 6 h, 2, 3, 5, 8, 11 days | 3.95 days | 1 day | 612 µg/mL | Bilateral injection | [30] | |||||
| 1.5 ng/mL | NC | AH | 2.63 days | 1 day | 478 µg/mL | |||||||||||
| 15.6 ng/mL | NC | Serum | 2, 6, 12, 24, 36, 48 h; 4–11 days | 3.47 days | 6 h | 616 µg/mL | ||||||||||
| Cynomolgus macaques | 0.25 mg/0.05 mL | ELISA | LLOD = 156 pg/mL | 1C | VH | 1, 3 days; 1–8 weeks | [56] | |||||||||
| AH | 2.3 days | 1 day | 51.3 µg/mL | 171 days ·µg/mL | 1.4 days | 41.8 µg/mL | 154 days ·µg/mL | |||||||||
| Serum | ||||||||||||||||
| Human | 0.5 mg/0.05 mL | ELISA | 10–1000 ng/mL | 1C | VH | 1–37 days | [32] | |||||||||
| AH | 7.19 days | 1 day | 56.1 µg/mL | |||||||||||||
| Serum | ||||||||||||||||
| Human | Variable | CLIA | LLOQ = 0.3 ng/mL | 1C | VH | Variable | 9 days | [22] | ||||||||
| AH | ||||||||||||||||
| Serum | 2 h | |||||||||||||||
| Human | 0.5 mg/0.05 mL | ELISA | LLOQ = 15 pg/mL | NC | VH | 3 h; 1, 3, 7, 28 days | [19,31] | |||||||||
| AH | ||||||||||||||||
| Serum | 5.8 days | 0.11 nM | 0.46 h·nM | |||||||||||||
| Model | Injected Dose | Determination | Sensitivity | PK Model | Sample | Time Points | Normal Eyes | Vitrectomy | Aphakia | Observations | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t1/2 | Tmax | Cmax | AUC | t1/2 | Cmax | AUC | t1/2 | |||||||||
| Dutch-belted rabbits | 1.25 mg/0.05 mL | CLIA | LLOQ = 0.0625 ng/mL | NC | VH | 1, 3, 8, 15, 29 days | 4.32 days | 1 day | 400 µg/mL | [41] | ||||||
| AH | 4.88 days | 3 days | 37.7 µg/mL | |||||||||||||
| Serum | 6.86 days | 8 days | 3.33 µg/mL | |||||||||||||
| Dutch-belted rabbits | 1.25 mg/0.05 mL | ELISA | LLOQ = 0.1 ng/mL | VH | 1, 2, 4, 12 weeks | 5.95 days | 7 days | 59.7308 µg/mL | [42] | |||||||
| AH | 7 days | 373.6 ng/mL | ||||||||||||||
| Serum | 12.95 days | 14 days | 2.0872 µg/mL | |||||||||||||
| Dutch-belted rabbits | 1.25 mg/0.05 mL | PET (I-124) | 2C | VH | 0, 2, 5, 7, 14, 21, 28, 35 days | 4.22 days | 0 h | No detection in other organs | [26] | |||||||
| AH | ||||||||||||||||
| Serum | ||||||||||||||||
| Dutch-belted rabbits | 1.25 mg/0.05 mL | PET (I-124) | 2C | VH | 0, 2, 5, 7, 14, 21, 28, 35 days | 4.22 days | 2.30 days | 2.08 days | [60] | |||||||
| AH | ||||||||||||||||
| Serum | ||||||||||||||||
| Dutch-belted rabbits | 1.25 mg/0.05 mL | ELISA | LLOD = 10 ng/mL | 1C | VH | 2, 4, 7, 10, 14, 21, 28, 35 days | [61] | |||||||||
| AH | ||||||||||||||||
| Serum | 6.69 days | 6.4 days | 6.22 µg/mL | 69.2 d·µg/mL | 2.80 days | 6.19 µg/mL | 84.1 d·µg/mL | 1.41 days | ||||||||
| New Zealand rabbits | 1.25 mg/0.05 mL | ELISA | LLOD = 0.01 ng/mL | NC | VH | 1, 3, 8, 15, 29 days | 6.61 days | 1 day | 406.25 µg/mL | [43] | ||||||
| AH | 6.51 days | 1 day | 5.835 µg/mL | |||||||||||||
| Serum | 5.87 days | 8 days | 0.413 µg/mL | |||||||||||||
| New Zealand rabbits | 1.25 mg/0.05mL | ELISA | LLOQ = 0.0625 ng/mL | 1C | VH | 1 h; 1, 2, 5, 14, 30 days | 7.06 days | 1 h | 1021.54 mg/mL | 6.99 days | [62] | |||||
| AH | 2 days | 121 mg/mL | ||||||||||||||
| Serum | ||||||||||||||||
| New Zealand rabbits | 0.025 mL | PET (Zr-89) | VH | 5–60 min; 4, 24, 48, 120, 144 h | 3.51 days | [63] | ||||||||||
| AH | ||||||||||||||||
| Serum | ||||||||||||||||
| Owl monkeys | 1.25 mg/0.05 mL | PET (I-124) | 2C | VH | 0, 1, 2, 4, 8, 14, 21, 28, 35 days | 3.60 days | [29] | |||||||||
| AH | ||||||||||||||||
| Serum | 1, 2, 4, 8, 12 h; 1, 2, 4, 8, 14, 21, 28, 35 days | 3.5 days | 7.80 ng/mL | |||||||||||||
| Cynomolgus macaques | 1.25 mg/0.05 mL | ELISA | LLOD = 0.156 ng/mL | VH | 1, 3, 7 d; 2, 4, 6, 8 weeks | [44] | ||||||||||
| AH | 2.8 days | 1 day | 49.500 µg/mL | |||||||||||||
| Serum | 12.3 days | 7 days | 1.430 µg/mL | |||||||||||||
| Cynomolgus macaques | 1.25 mg/0.05 mL | ELISA | 7.8–1000 pg/mL | VH | 1, 3, 7 d; 2, 4, 6, 8 weeks | [64] | ||||||||||
| AH | 1 day | 10.8 μg/mL | 1.5 days | |||||||||||||
| Serum | 5.9 days | 1 day | 42.2 ng/mL | |||||||||||||
| Human | 1.25 mg/0.05 mL | ELISA | LLOQ = 313 pg/mL | NC | VH | 3 h; 1, 3, 7, 28 days | [19,31] | |||||||||
| AH | ||||||||||||||||
| Serum | 18.7 days | 0.76 nM | 16.10 h·nM | |||||||||||||
| Human | 1.25 mg/0.05 mL | ELISA | 2C | VH | 1–101 days | 6.7 days | 2 days | 165 µg/mL | 2036 d·µg/mL | [47] | ||||||
| AH | ||||||||||||||||
| Serum | ||||||||||||||||
| Human | 1.25 mg/0.05 mL | ELISA | 6.25 ng/mL | VH | Variable | 4.9 days | 0.66 day | [34] | ||||||||
| AH | ||||||||||||||||
| Serum | 11.3 days | |||||||||||||||
| Human | 1.5 mg | ELISA | 1C | VH | 1–53 days | [52] | ||||||||||
| AH | 9.82 days | 1 day | 33.3 µg/mL | |||||||||||||
| Serum | ||||||||||||||||
| Human | 1.5 mg | ELISA | 1C | VH | 1–60 days | [53] | ||||||||||
| AH | 7.85 days | 1 day | 14.86 µg/mL | |||||||||||||
| Serum | ||||||||||||||||
| Human | 3 mg | ELISA | 1C | VH | 1–60 days | [53] | ||||||||||
| AH | 11.69 days | 1 day | 27.74 µg/mL | |||||||||||||
| Serum | ||||||||||||||||
| Model | Injected dose | Determination | Sensitivity | PK Model | Sample | Time Points | Normal Eyes | Vitrectomy | Aphakia | Observations | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t1/2 | Tmax | Cmax | AUC | t1/2 | Cmax | AUC | t1/2 | |||||||||
| Dutch-belted rabbits | 2 mg/0.05 mL | PET (I-124) | 1C | VH | 0, 2, 5, 7, 14, 21, 28, 35 days | 4.58 days | 0 h | No detection in other organs | [45] | |||||||
| AH | ||||||||||||||||
| Serum | ||||||||||||||||
| Owl monkeys | 2 mg/0.05 mL | PET (I-124) | 2C | VH | 0, 1, 2, 4, 8, 14, 21, 28, 35 days | 2.44 days | [29] | |||||||||
| AH | ||||||||||||||||
| Serum | 1, 2, 4, 8, 12 h; 1, 2, 4, 8, 14, 21, 28, 35 days | 2 days | 3.50 ng/mL | |||||||||||||
| Cynomolgus macaques | 2 mg/0.05 mL | ELISA | LLOD = 156 pg/mL | 1C | VH | 1, 3 days; 1–8 weeks | [56] | |||||||||
| AH | 2.2 days | 1 day | 74 µg/mL | 174 d·µg/mL | 1.5 days | 68 µg/mL | 124 d·µg/mL | |||||||||
| Serum | ||||||||||||||||
| Human | 2 mg/0.05 mL | ELISA | LLOQ = 1000 pg/mL | NC | VH | 3 h; 1, 3, 7, 28 days | [19,31] | |||||||||
| AH | ||||||||||||||||
| Serum | 11.4 days | 0.45 nM | 4.32 h·nM | |||||||||||||
| Human | 2 mg | ELISA | VH | 4 h; 1, 3, 7, 14, 28 days | [59] | |||||||||||
| AH | 11 days | 4 h | 64.4 mg/L | |||||||||||||
| Serum | 4 h | 0 mg/L | ||||||||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Quintanilla, L.; Luaces-Rodríguez, A.; Gil-Martínez, M.; Mondelo-García, C.; Maroñas, O.; Mangas-Sanjuan, V.; González-Barcia, M.; Zarra-Ferro, I.; Aguiar, P.; Otero-Espinar, F.J.; et al. Pharmacokinetics of Intravitreal Anti-VEGF Drugs in Age-Related Macular Degeneration. Pharmaceutics 2019, 11, 365. https://doi.org/10.3390/pharmaceutics11080365
García-Quintanilla L, Luaces-Rodríguez A, Gil-Martínez M, Mondelo-García C, Maroñas O, Mangas-Sanjuan V, González-Barcia M, Zarra-Ferro I, Aguiar P, Otero-Espinar FJ, et al. Pharmacokinetics of Intravitreal Anti-VEGF Drugs in Age-Related Macular Degeneration. Pharmaceutics. 2019; 11(8):365. https://doi.org/10.3390/pharmaceutics11080365
Chicago/Turabian StyleGarcía-Quintanilla, Laura, Andrea Luaces-Rodríguez, María Gil-Martínez, Cristina Mondelo-García, Olalla Maroñas, Víctor Mangas-Sanjuan, Miguel González-Barcia, Irene Zarra-Ferro, Pablo Aguiar, Francisco J. Otero-Espinar, and et al. 2019. "Pharmacokinetics of Intravitreal Anti-VEGF Drugs in Age-Related Macular Degeneration" Pharmaceutics 11, no. 8: 365. https://doi.org/10.3390/pharmaceutics11080365
APA StyleGarcía-Quintanilla, L., Luaces-Rodríguez, A., Gil-Martínez, M., Mondelo-García, C., Maroñas, O., Mangas-Sanjuan, V., González-Barcia, M., Zarra-Ferro, I., Aguiar, P., Otero-Espinar, F. J., & Fernández-Ferreiro, A. (2019). Pharmacokinetics of Intravitreal Anti-VEGF Drugs in Age-Related Macular Degeneration. Pharmaceutics, 11(8), 365. https://doi.org/10.3390/pharmaceutics11080365