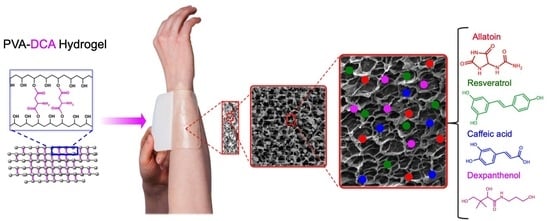
Film Dressings Based on Hydrogels: Simultaneous and Sustained-Release of Bioactive Compounds with Wound Healing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theoretical Section
2.1.1. Building Molecular Structures
2.1.2. In-Silico Calculation of Interaction Energies
2.2. Experimental Section
2.2.1. Materials
2.2.2. Synthesis of Selected Hydrogels Based on PVA, Dicarboxylic Acids and Bioactive Compound Loading
2.2.3. Equilibrium Swelling Ratio of PDCAH
2.2.4. Infrared Spectroscopy
2.2.5. Mechanical analysis
2.2.6. Scanning Electron Microscopy Analysis
2.2.7. Sustained Release Kinetics of BC from PDCAH-BC
2.2.8. Wound Healing Testing on Dermal Models of Rats
Animals and Maintenance Conditions
Experimental Procedure
2.2.9. Histological Analysis
2.2.10. Cytotoxicity and Cell Viability
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. In-Silico Interaction Energy Study
3.2. Preparation of PDCAH
3.3. ESR Results
3.4. BC in Vitro Release Behavior of Supramolecular PDCAH
3.5. FTIR Analysis
3.6. Mechanical Analysis
3.7. SEM Analysis
3.8. In Vivo Wound Healing Studies
3.9. Histological Analysis
3.10. PDCAH Cytotoxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kondo, T.; Ishida, Y. Molecular pathology of wound healing. Forensic Sci. Int. 2010, 203, 93–98. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.L.; Lee, M.H.; You, K.E.; Kwon, B.J.; Seo, H.J.; Park, J.C. Asiaticoside enhances normal human skin cell migration, attachment and growth in vitro wound healing model. Phytomedicine 2012, 19, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Araújo, L.U.; Grabe-Guimarães, A.; Mosqueira, V.C.F.; Carneiro, C.M.; Silva-Barcellos, N.M. Profile of wound healing process induced by allantoin. Acta Cir. Bras. 2010, 25, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Croft, C.; Tarin, D. Ultrastructural studies of wound healing in mouse skin. J. Anat. 1970, 106, 63–77. [Google Scholar]
- Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C.; Teot, L.; Vanscheidt, W. Wound bed preparation: A systematic approach to wound management. Wound Repair Regen. 2003, 11, S1–S28. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Kamoun, E.; Kenawy, E.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Lee, K.J.; Kim, S.I. Thermo-sensitive Swelling Behavior of Poly(2-Ethyl-2-oxazoline)/Poly(Vinyl Alcohol) Interpenetrating Polymer Network Hydrogels. J. Macromol. Sci. A 2004, 41, 267–274. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Nuñez, Y.A.; Castro, R.I.; Arenas, F.A.; López-Cabaña, Z.E.; Carreño, G.; Carrasco-Sánchez, V.; Marican, A.; Villaseñor, J.; Vargas, E.; Santos, L.S.; et al. Preparation of Hydrogel/Silver Nanohybrids Mediated by Tunable-Size Silver Nanoparticles for Potential Antibacterial Applications. Polymers 2019, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Grijalvo, S.; Mayr, J.; Eritja, R.; Díaz, D.D. Biodegradable liposome-encapsulated hydrogels for biomedical applications: A marriage of convenience. Biomater. Sci. UK 2016, 4, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.P.; Espiga, A.; Silva, D.; Baptista, P.; Henriques, J.; Ferreira, C.; Silva, J.C.; Borges, J.P.; Pires, E.; Chaves, P.; et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009, 17, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, R.N.; McGuinness, G.B.; Ramos, M.E.; Kajiyama, C.E.; Thiré, R.M. Properties of PVA Hydrogel Wound-Care Dressings Containing UK Propolis. Macromol. Symp. 2016, 368, 122–127. [Google Scholar] [CrossRef]
- León, K.; Santiago, J. Propiedades antimicrobianas de películas de quitosano-alcohol polivinílico embebidas en extracto de sangre de grado. Rev. Soc. Quim. Perú 2007, 73, 158–165. [Google Scholar]
- Marican, A.; Avila-Salas, F.; Valdés, O.; Wehinger, S.; Villaseñor, J.; Fuentealba, N.; Arenas-Salinas, M.; Argandoña, Y.; Carrasco-Sánchez, V.; Durán-Lara, E.F. Rational Design, Synthesis and Evaluation of γ-CD-Containing Cross-Linked Polyvinyl Alcohol Hydrogel as a Prednisone Delivery Platform. Pharmaceutics 2018, 10, 30. [Google Scholar] [CrossRef]
- Schanuel, F.S.; Santos, K.S.R.; Monte-Alto-Costa, A.; de Oliveira, M.G. Combined nitric oxide-releasing poly (vinyl alcohol) film/F127 hydrogel for accelerating wound healing. Colloids Surf. B 2015, 130, 182–191. [Google Scholar] [CrossRef]
- Chavda, H.V.; Patel, C.N. Effect of crosslinker concentration on characteristics of superporous hydrogel. Int. J. Pharm. Investig. 2011, 1, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.; Nguyen, S.; Nave, F. Characterization of PVA-IDA Hydrogel Crosslinked with 1.25%, 2.5% and 5% Glutaraldehyde. J. Chem. 2013, 1, 1–7. [Google Scholar] [CrossRef]
- Orienti, I.; Treré, R.; Luppi, B.; Bigucci, F.; Cerchiara, T.; Zuccari, G.; Zecchi, V. Hydrogels Formed by Crosslinked Poly (vinyl alcohol) as Sustained Drug Delivery Systems. Arch. Pharm. 2002, 2, 89–93. [Google Scholar] [CrossRef]
- Vrana, N.E.; Liu, Y.; McGuiness, G.B.; Cahill, P.A. Characterization of Poly (vinyl alcohol)/Chitosan Hydrogels as Vascular Tissue Engineering Scaffolds. Macromol. Symp. 2008, 269, 106–110. [Google Scholar] [CrossRef]
- Peng, Z.; Li, Z.; Zhang, F.; Peng, X. Preparation and Properties of Polyvinyl Alcohol/Collagen Hydrogel. J. Macromol. Sci. B 2012, 51, 1934–1941. [Google Scholar] [CrossRef]
- Chang, C.; Lue, A.; Zhang, L. Effects of Crosslinking Methods on Structure and Properties of Cellulose/PVA Hydrogels. Macromol. Chem. Phys. 2008, 209, 1266–1273. [Google Scholar] [CrossRef]
- Liu, Y.; Vrana, N.E.; Cahill, P.A.; McGuinness, G.B. Physically Crosslinked Composite Hydrogels of PVA With Natural Macromolecules: Structure, Mechanical Properties, and Endothelial Cell Compatibility. J. Biomed. Mater. Res. B 2009, 90, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.F.; Campos, F.S.; Lage, A.P.; Leite, R.C.; Heneine, L.G.; Vasconcelos, W.L.; Lobato, Z.I.; Mansur, H.S. Synthesis and Characterization of Poly(Vinyl Alcohol) Hydrogels and Hybrids for rMPB70 Protein Adsorption. Mater. Res. 2006, 9, 185–191. [Google Scholar] [CrossRef]
- Roberts, J.; Farrugia, B.; Green, R.; Rnjak-Kovacina, J.; Martens, P. In situ formation of poly (vinyl alcohol)–heparin hydrogels for mild encapsulation and prolonged release of basic fibroblast growth factor and vascular endothelial growth factor. J. Tissue Eng. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Singh, B.; Pal, L. Sterculia crosslinked PVA and PVA-poly(AAm) hydrogel wound dressings for slow drug delivery: Mechanical, mucoadhesive, biocompatible and permeability properties. J. Mech. Behav. Biomed. 2012, 9, 9–21. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef]
- Pirinen, S.; Karvinen, J.; Tiitu, V.; Suvanto, M.; Pakkanen, T.T. Control of swelling properties of polyvinyl alcohol/hyaluronic acid hydrogels for the encapsulation of chondrocyte cells. J. Appl. Polym. Sci. 2015, 132, 28. [Google Scholar] [CrossRef]
- Valderruten, N.E.; Valverde, J.D.; Zuluaga, F.; Durántez-Ruiz, E. Synthesis and characterization of chitosan hydrogels cross-linked with dicarboxylic acids. React. Funct. Polym. 2015, 84, 21–28. [Google Scholar] [CrossRef]
- US Food and Drug Administration Guidance for Industry: Co-development of two or more new investigational drugs for use in combination. June 2013. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/codevelopment-two-or-more-new-investigational-drugs-use-combination (accessed on 28 July 2019).
- Wu, M.; Sirota, M.; Butte, A.J.; Chen, B. Characteristics of drug combination therapy in oncology by analyzing clinical trial data on clinicaltrials.gov. Pac. Symp. Biocomput. 2015, 68–79. [Google Scholar]
- Bouhadir, K.H.; Alsberg, E.; Mooney, D.J. Hydrogels for combination delivery of antineoplastic agents. Biomaterials 2001, 22, 2625–2633. [Google Scholar] [CrossRef]
- Singh, B.; Dhimana, A. Designing bio-mimetic moxifloxacin loaded hydrogel wound dressing to improve antioxidant and pharmacology properties. RSC Adv. 2015, 5, 44666–44678. [Google Scholar] [CrossRef]
- Hassan, A.; Niazi, M.B.K.; Hussain, A.; Farrukh, S.; Ahmad, T. Development of anti-bacterial PVA/starch based hydrogel membrane for wound dressing. J. Polym. Environ. 2018, 26, 235–243. [Google Scholar] [CrossRef]
- ChemAxon Ltd. MarvinSketch Program Version 19.01 (For OSX); ChemAxon Ltd.: Budapest, Hungary, 2019; Available online: https://chemaxon.com/products/marvin (accessed on 20 June 2018).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.J.; Vreven, T.; Kudin, K.; Burant, J. Gaussian 16, Revision, A.03, Gaussian Inc.: Wallingford, CT, USA, 2016.
- Fan, C.F.; Olafson, B.D.; Blanco, M. Application of molecular simulation to derive phase diagrams of binary mixtures. Macromolecules 1992, 25, 3667–3676. [Google Scholar] [CrossRef]
- Avila-Salas, F.; Sandoval, C.; Caballero, J.; Guiñez-Molinos, S.; Santos, L.S.; Cachau, R.E.; González-Nilo, F.D. Study of Interaction Energies Between the PAMAM Dendrimer and Nonsteroidal Anti-inflammatory Drug Using a Distributed Computational Strategy and Experimental Analysis by ESI-MS/MS. J. Phys. Chem. B 2012, 116, 2031–2039. [Google Scholar] [CrossRef]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.I.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1984, 65, 55–63. [Google Scholar] [CrossRef]
- Gupta, N.V.; Shivakumar, H.G. Investigation of Swelling Behavior and Mechanical Properties of a pH-Sensitive Superporous Hydrogel Composite. Iran. J Pharm. Res. 2012, 11, 481–493. [Google Scholar] [PubMed]
- Avila-Salas, F.; Rodriguez Nuñez, Y.A.; Marican, A.; Castro, R.I.; Villaseñor, J.; Santos, L.S.; Wehinger, S.; Durán-Lara, E.F. Rational Development of a Novel Hydrogel as a pH-Sensitive Controlled Release System for Nifedipine. Polymers 2018, 10, 806. [Google Scholar] [CrossRef] [PubMed]
- Kristin, E.; Curtis, W.F. Protein diffusion in photopolymerized poly(ethylene glycol) hydrogel networks. Biomed. Mater. 2011, 6, 055006. [Google Scholar]
- Masri, C.; Chagnon, G.; Favier, D. Influence of processing parameters on the macroscopic mechanical behavior of PVA hydrogels. Mater. Sci. Eng. C 2017, 75, 769–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiellini, E.; Cinelli, P.; Imam, S.H.; Mao, L. Composite films based on biorelated agro-industrial waste and poly(vinyl alcohol). preparation and mechanical pxroperties characterization. Biomacromolecules 2001, 2, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Heise, R.; Skazik, C.; Marquardt, Y.; Czaja, K.; Sebastian, K.; Kurschat, P.; Gan, L.; Denecke, B.; Ekanayake-Bohlig, S.; Wilhelm, K.-P.; et al. Dexpanthenol modulates gene expression in skin wound healing in vivo. Skin Pharmacol. Physiol. 2012, 25, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Tsai, Y.F.; Tsai, H.I.; Yu, H.P. Anti-inflammatory and organ-protective effects of resveratrol in trauma-hemorrhagic injury. Mediat. Inflamm. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Assadian, O.; Koburger-Janssen, T. Antimicrobial efficacy of the combination of chlorhexidine digluconate and dexpanthenol. GMS Hyg. Infect. Control 2016, 11, 1–6. [Google Scholar]
- Natarajan, K.; Singh, S.; Burke, T.R.; Grunberger, D.; Aggarwal, B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kB. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar] [CrossRef]
- Yaman, I.; Derici, H.; Kara, C.; Kamer, E.; Diniz, G.; Ortac, R.; Sayin, O. Effects of resveratrol on incisional wound healing in rats. Surge. Today 2013, 43, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Kadoma, Y.; Fujisawa, S. Radical-scavenging activity of dietary phytophenols in combination with co-antioxidants using the induction period method. Molecules 2011, 16, 10457–10470. [Google Scholar] [CrossRef] [PubMed]








| PDCAH | Crosslinker | Crosslinker Ratio * | Bioactive Compounds | |||
|---|---|---|---|---|---|---|
| Allantoin * | Caffeic Acid * | Resveratrol * | Dexpanthenol * | |||
| PMALEH | Male | 20 | 5 | 2 | 2 | 2 |
| PAAH | AA | 20 | 5 | 2 | 2 | 2 |
| PMALIH | Mali | 20 | 5 | 2 | 2 | 2 |
| PSAH | SA | 20 | 5 | 2 | 2 | 2 |
| Id. | Hydrogel Nanopores (Hnp) | HNP-AL ΔE Kcal/mol | HNP-RES ΔE Kcal/mol | HNP-CA ΔE Kcal/mol | NPH-DEX ΔE Kcal/mol | Average ΔE Kcal/mol |
|---|---|---|---|---|---|---|
| 1 | PVAnp-Oxalic acid | −3.049 | −2.090 | −0.589 | −1.780 | −1.877 |
| 2 | PVAnp-Malonic acid | −3.083 | −2.093 | −0.876 | −1.824 | −1.969 |
| 3 | PVAnp-Succinic acid | −3.187 * | −2.172 * | −0.962 * | −1.963 * | −2.071 * |
| 4 | PVAnp-Malic acid | −3.127 * | −2.149 * | −0.986 * | −1.933 * | −2.049 * |
| 5 | PVAnp-Fumaric acid | −3.012 | −2.049 | −0.873 | −1.833 | −1.942 |
| 6 | PVAnp-Maleic acid | −3.153 * | −2.156 * | −0.950 * | −1.964 * | −2.056 * |
| 7 | PVAnp-Citraconic acid | −3.052 | −2.093 | −0.857 | −1.783 | −1.946 |
| 8 | PVAnp-Itaconic acid | −3.069 | −2.079 | −0.862 | −1.810 | −1.955 |
| 9 | PVAnp-Tartaric acid | −3.093 | −2.078 | −0.868 | −1.769 | −1.952 |
| 10 | PVAnp-Glutaric acid | −3.095 | −2.117 | −0.905 | −1.839 | −1.989 |
| 11 | PVAnp-Adipic acid | −3.089 | −2.105 | −0.868 | −1.791 | −1.963 |
| 12 | PVAnp-Pimelic acid | −3.009 | −2.012 | −0.813 | −1.720 | −1.889 |
| 13 | PVAnp-Suberic acid | −3.032 | −2.073 | −0.572 | −1.763 | −1.860 |
| 14 | PVAnp-Azelaic acid | −3.079 | −2.089 | −0.872 | −1.820 | −1.965 |
| 15 | PVAnp-Phtalic acid | −3.108 | −2.093 | −0.883 | −1.784 | −1.967 |
| 16 | PVAnp-Isophtalic acid | −3.114 | −2.136 | −0.924 | −1.858 | −2.008 |
| 17 | PVAnp-Terephtalic acid | −3.125 | −2.141 | −0.933 | −1.906 | −2.026 |
| 18 | PVAnp-2,5-pyridin acid | −3.117 | −2.079 | −0.842 | −1.858 | −1.982 |
| 19 | PVAnp-Aspartic acid | −3.130 * | −2.150 * | −0.951 * | −1.910 * | −2.035 * |
| 20 | PVAnp-Glutamic acid | −3.063 | −2.133 | −0.905 | −1.874 | −1.994 |
| Hydrogel | Rapid-Release Phase of BC (%) until 6 h | |||
|---|---|---|---|---|
| Allantoin | Dexpanthenol | Caffeic Acid | Resveratrol | |
| PMALEH-BC | 35 ± 4.1 | 38 ± 2.0 | 52 ± 1.2 | 56 ± 2.0 |
| PAAH-BC | 35 ± 1.7 | 32 ± 3.0 | 50 ± 5.5 | 58 ± 2.6 |
| PMALIH-BC | 58 ± 2.6 | 47 ± 4.9 | 32 ± 3.0 | 38 ± 2.0 |
| PSAH-BC | 58 ± 2.6 | 46 ± 3.0 | 38 ± 2.0 | 32 ± 3.0 |
| Hydrogel | Rapid-Release Phase | |||
| Allantoin | Dexpanthenol | |||
| mg/6 h | mg/h | mg/6 h | mg/h | |
| PMALEH-BC | 7.0 ± 0.82 | 1.17 ± 0.14 | 7.6 ± 0.4 | 1.27 ± 0.07 |
| PAAH-BC | 6.4 ± 0.34 | 1.07 ± 0.06 | 6.4 ± 0.6 | 1.07 ± 0.10 |
| PMALIH-BC | 9.4 ± 0.52 | 1.57 ± 0.09 | 9.4 ± 1.0 | 1.57 ± 0.16 |
| PSAH-BC | 9.2 ± 0.52 | 1.53 ± 0.09 | 9.2 ± 0.6 | 1.53 ± 0.10 |
| Hydrogel | Caffeic Acid | Caffeic acid | ||
| mg/6 h | mg/h | mg/6 h | mg/h | |
| PMALEH-BC | 10.4 ± 0.24 | 1.73 ± 0.04 | 11.2 ± 0.4 | 1.87 ± 0.07 |
| PAAH-BC | 10.0 ± 1.1 | 1.67 ± 0.18 | 11.6 ± 0.52 | 1.93 ± 0.09 |
| PMALIH-BC | 6.4 ± 0.60 | 1.07 ± 0.10 | 7.6 ± 0.40 | 1.27 ± 0.07 |
| PSAH-BC | 7.6 ± 0.40 | 1.27 ± 0.07 | 6.4 ± 0.60 | 1.07 ± 0.10 |
| Hydrogel | Slow-Release Phase | |||
| Allantoin | Dexpanthenol | |||
| mg/114 h | mg/h | mg/114 h | mg/h | |
| PMALEH-BC | 13.0 ± 0.50 | 0.11 ± 0.00 | 12.4 ± 0.40 | 0.11 ± 0.00 |
| PAAH-BC | 13.0 ± 0.58 | 0.11 ± 0.01 | 13.6 ± 0.48 | 0.12 ± 0.00 |
| PMALIH-BC | 8.4 ± 0.58 | 0.07 ± 0.01 | 10.6 ± 1.10 | 0.09 ± 0.01 |
| PSAH-BC | 8.4 ± 0.64 | 0.07 ± 0.01 | 10.8 ± 0.80 | 0.09 ± 0.01 |
| Hydrogel | Caffeic Acid | Resveratrol | ||
| mg/114 h | mg/h | mg/114 h | mg/h | |
| PMALEH-BC | 9.6 ± 0.50 | 0.08 ± 0.00 | 8.8 ± 0.62 | 0.08 ± 0.01 |
| PAAH-BC | 10.0 ± 0.76 | 0.09 ± 0.01 | 8.4 ± 0.70 | 0.07 ± 0.01 |
| PMALIH-BC | 13.6 ± 0.58 | 0.12 ± 0.01 | 12.4 ± 0.40 | 0.11 ± 0.00 |
| PSAH-BC | 12.4 ± 0.4 | 0.11 ± 0.00 | 13.6 ± 0.88 | 0.12 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Salas, F.; Marican, A.; Pinochet, S.; Carreño, G.; Valdés, O.; Venegas, B.; Donoso, W.; Cabrera-Barjas, G.; Vijayakumar, S.; Durán-Lara, E.F. Film Dressings Based on Hydrogels: Simultaneous and Sustained-Release of Bioactive Compounds with Wound Healing Properties. Pharmaceutics 2019, 11, 447. https://doi.org/10.3390/pharmaceutics11090447
Ávila-Salas F, Marican A, Pinochet S, Carreño G, Valdés O, Venegas B, Donoso W, Cabrera-Barjas G, Vijayakumar S, Durán-Lara EF. Film Dressings Based on Hydrogels: Simultaneous and Sustained-Release of Bioactive Compounds with Wound Healing Properties. Pharmaceutics. 2019; 11(9):447. https://doi.org/10.3390/pharmaceutics11090447
Chicago/Turabian StyleÁvila-Salas, Fabian, Adolfo Marican, Soledad Pinochet, Gustavo Carreño, Oscar Valdés, Bernardo Venegas, Wendy Donoso, Gustavo Cabrera-Barjas, Sekar Vijayakumar, and Esteban F. Durán-Lara. 2019. "Film Dressings Based on Hydrogels: Simultaneous and Sustained-Release of Bioactive Compounds with Wound Healing Properties" Pharmaceutics 11, no. 9: 447. https://doi.org/10.3390/pharmaceutics11090447
APA StyleÁvila-Salas, F., Marican, A., Pinochet, S., Carreño, G., Valdés, O., Venegas, B., Donoso, W., Cabrera-Barjas, G., Vijayakumar, S., & Durán-Lara, E. F. (2019). Film Dressings Based on Hydrogels: Simultaneous and Sustained-Release of Bioactive Compounds with Wound Healing Properties. Pharmaceutics, 11(9), 447. https://doi.org/10.3390/pharmaceutics11090447