3D Printing of Drug-Loaded Thermoplastic Polyurethane Meshes: A Potential Material for Soft Tissue Reinforcement in Vaginal Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Thermoplastic Poly(urethane) (TPU) Filaments Containing Levofloxacin (LFX)
2.3. Preparation of 3D Printed Meshes Containing LFX
2.4. Characterization of 3D Printed Meshes
2.4.1. Mechanical Properties
2.4.2. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. Scanning Electron Microscopy (SEM)
2.4.5. X-ray Microcomputed Tomography (μCT)
2.5. In Vitro Drug Release Studies
2.6. In Vitro Microbiological Analysis
2.7. Statistical Analysis
3. Results
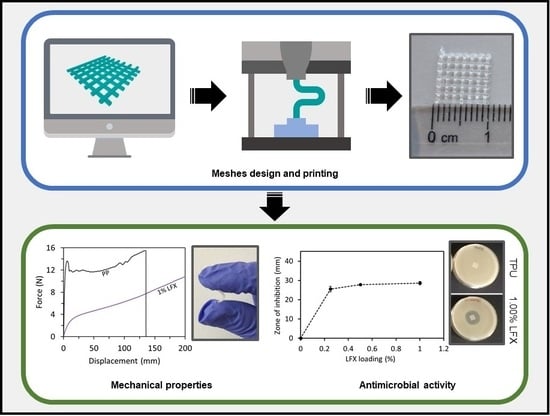
3.1. Preparation and Characterisation of TPU Filaments and Meshes Containing LFX
3.2. Mechanical Characterisation of LFX 3D Printed Meshes
3.3. LFX Release from 3D Printed Meshes
3.4. Antimicrobial Properties of LFX Loaded 3D Printed Meshes
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Y.M.; Welk, B. Revisiting current treatment options for stress urinary incontinence and pelvic organ prolapse: A contemporary literature review. Res. Rep. Urol. 2019, 11, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Mangir, N.; Chapple, C.R.; MacNeil, S. Synthetic Materials Used in the Surgical Treatment of Pelvic Organ Prolapse: Problems of Currently Used Material and Designing the Ideal Material. In Pelvic Floor Disorders; Rizvi, R., Ed.; InTechOpen: London, UK, 2018; Volume i, p. 13. ISBN 978-1-78-923245-5. [Google Scholar]
- Vergeldt, T.F.M.; Weemhoff, M.; IntHout, J.; Kluivers, K.B. Risk factors for pelvic organ prolapse and its recurrence: A systematic review. Int. Urogynecol. J. 2015, 26, 1559–1573. [Google Scholar] [CrossRef] [Green Version]
- Niaounakis, M. Medical, Dental, and Pharmaceutical Applications. In Biopolymers: Applications and Trends; Niaounakis, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 291–405. ISBN 978-0-32-335399-1. [Google Scholar]
- Mironska, E.; Chapple, C.; MacNeil, S. Recent advances in pelvic floor repair. F1000Research 2019, 8, 778. [Google Scholar] [CrossRef] [Green Version]
- Rac, G.; Younger, A.; Clemens, J.Q.; Kobashi, K.; Khan, A.; Nitti, V.; Jacobs, I.; Lemack, G.E.; Brown, E.T.; Dmochowski, R.; et al. Stress urinary incontinence surgery trends in academic female pelvic medicine and reconstructive surgery urology practice in the setting of the food and drug administration public health notifications. Neurourol. Urodyn. 2017, 36, 1155–1160. [Google Scholar] [CrossRef]
- The Food and Drug Administration. Obstetrical and Gynecological Devices; Reclassification of Surgical Mesh for Transvaginal Pelvic Organ Prolapse Repair; Final Order. Fed. Regist. 2016, 81, 353–361. [Google Scholar]
- Mancuso, E.; Downey, C.; Doxford-Hook, E.; Bryant, M.G.; Culmer, P. The use of polymeric meshes for pelvic organ prolapse: Current concepts, challenges, and future perspectives. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019. [Google Scholar] [CrossRef]
- FitzGerald, J.; Kumar, A. Biologic versus Synthetic Mesh Reinforcement: What are the Pros and Cons? Clin. Colon Rectal Surg. 2014, 27, 140–148. [Google Scholar]
- Hympánová, L.; Rynkevic, R.; Román, S.; Mori da Cunha, M.G.M.C.; Mazza, E.; Zündel, M.; Urbánková, I.; Gallego, M.R.; Vange, J.; Callewaert, G.; et al. Assessment of Electrospun and Ultra-lightweight Polypropylene Meshes in the Sheep Model for Vaginal Surgery. Eur. Urol. Focus 2018. [Google Scholar] [CrossRef] [Green Version]
- De Tayrac, R.; Chentouf, S.; Garreau, H.; Braud, C.; Guiraud, I.; Boudeville, P.; Vert, M. In vitro degradation and in vivo biocompatibility of poly(lactic acid) mesh for soft tissue reinforcement in vaginal surgery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85, 529–536. [Google Scholar] [CrossRef]
- Shafaat, S.; Mangir, N.; Regureos, S.R.; Chapple, C.R.; MacNeil, S. Demonstration of improved tissue integration and angiogenesis with an elastic, estradiol releasing polyurethane material designed for use in pelvic floor repair. Neurourol. Urodyn. 2018, 37, 716–725. [Google Scholar] [CrossRef]
- Hillary, C.J.; Roman, S.; Bullock, A.J.; Green, N.H.; Chapple, C.R.; MacNeil, S. Developing Repair Materials for Stress Urinary Incontinence to Withstand Dynamic Distension. PLoS ONE 2016, 11, e0149971. [Google Scholar] [CrossRef] [Green Version]
- Mathew, E.; Domínguez-Robles, J.; Stewart, S.; Mancuso, E.; O’Donnell, K.; Larraneta, E.; Lamprou, D.A. Fused Deposition Modelling as an Effective Tool for Anti-Infective Dialysis Catheter Fabrication. ACS Biomater. Sci. Eng. 2019, 5, 6300–6310. [Google Scholar] [CrossRef]
- Mathew, E.; Domínguez-Robles, J.; Larrañeta, E.; Lamprou, D.A. Fused Deposition Modelling as a Potential Tool for Antimicrobial Dialysis Catheters Manufacturing: New Trends vs. Conventional Approaches. Coatings 2019, 9, 515. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.; Brambilla, D.; Leroux, J.-C. Is 3D Printing of Pharmaceuticals a Disruptor or Enabler? Adv. Mater. 2019, 31, 1805680. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Madla, C.M.; Hatton, G.B.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the future: Recent advances of 3D printing in drug delivery and healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094. [Google Scholar] [CrossRef]
- Stewart, S.; Domínguez-Robles, J.; Donnelly, R.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Robles, J.; Martin, N.; Fong, M.; Stewart, S.; Irwin, N.; Rial-Hermida, M.; Donnelly, R.; Larrañeta, E. Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- Turner, B.N.; Strong, R.; Gold, S.A. A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyp. J. 2014, 20, 192–204. [Google Scholar] [CrossRef]
- Matos, B.D.M.; Rocha, V.; da Silva, E.J.; Moro, F.H.; Bottene, A.C.; Ribeiro, C.A.; dos Santos Dias, D.; Antonio, S.G.; do Amaral, A.C.; Cruz, S.A.; et al. Evaluation of commercially available polylactic acid (PLA) filaments for 3D printing applications. J. Therm. Anal. Calorim. 2019, 137, 555–562. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Larrañeta, E.; Fong, M.L.; Martin, N.K.; Irwin, N.J.; Mutjé, P.; Tarrés, Q.; Delgado-Aguilar, M. Lignin/poly(butylene succinate) composites with antioxidant and antibacterial properties for potential biomedical applications. Int. J. Biol. Macromol. 2020, 145, 92–99. [Google Scholar] [CrossRef]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-specific 3D scanned and 3D printed antimicrobial polycaprolactone wound dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zheng, W.; Kodur, V.; Sun, H. Effect of temperature on mechanical properties of prestressing bars. Constr. Build. Mater. 2014, 61, 24–32. [Google Scholar] [CrossRef]
- Afonso, J.S.; Martins, P.A.L.S.; Girao, M.J.B.C.; Natal Jorge, R.M.; Ferreira, A.J.M.; Mascarenhas, T.; Fernandes, A.A.; Bernardes, J.; Baracat, E.C.; Rodrigues de Lima, G.; et al. Mechanical properties of polypropylene mesh used in pelvic floor repair. Int. Urogynecol. J. 2008, 19, 375–380. [Google Scholar] [CrossRef]
- Hall Barrientos, I.J.; Paladino, E.; Brozio, S.; Passarelli, M.K.; Moug, S.; Black, R.A.; Wilson, C.G.; Lamprou, D.A. Fabrication and characterisation of drug-loaded electrospun polymeric nanofibers for controlled release in hernia repair. Int. J. Pharm. 2017, 517, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Kruger, J.A.; Jor, J.W.Y.; Wong, V.; Dietz, H.P.; Nash, M.P.; Nielsen, P.M.F. Characterizing the ex vivo mechanical properties of synthetic polypropylene surgical mesh. J. Mech. Behav. Biomed. Mater. 2014, 37, 48–55. [Google Scholar] [CrossRef]
- Bako, A.; Dhar, R. Review of synthetic mesh-related complications in pelvic floor reconstructive surgery. Int. Urogynecol. J. 2009, 20, 103–111. [Google Scholar] [CrossRef]
- Urogynecologic Surgical Mesh: Update on the Safety and Effectiveness of Transvaginal Placement for Pelvic Organ Prolapse; The Food and Drug Administration (FDA): Hampton, VA, USA, 2011.
- Mangir, N.; Roman, S.; Chapple, C.R.; MacNeil, S. Complications related to use of mesh implants in surgical treatment of stress urinary incontinence and pelvic organ prolapse: Infection or inflammation? World J. Urol. 2019. [Google Scholar] [CrossRef]
- Weisman, J.A.; Nicholson, J.C.; Tappa, K.; Jammalamadaka, U.; Wilson, C.G.; Mills, D.K. Antibiotic and chemotherapeutic enhanced three-dimensional printer filaments and constructs for biomedical applications. Int. J. Nanomed. 2015, 10, 357–370. [Google Scholar]
- Tappa, K.; Jammalamadaka, U.; Weisman, J.; Ballard, D.; Wolford, D.; Pascual-Garrido, C.; Wolford, L.; Woodard, P.; Mills, D. 3D Printing Custom Bioactive and Absorbable Surgical Screws, Pins, and Bone Plates for Localized Drug Delivery. J. Funct. Biomater. 2019, 10, 17. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Nirmala, R.; Lee, J.Y.; Rahman, M.; Hong, S.-T.; Kim, H.Y. Antibacterial ciprofloxacin HCl incorporated polyurethane composite nanofibers via electrospinning for biomedical applications. Ceram. Int. 2013, 39, 4937–4944. [Google Scholar] [CrossRef]
- Naik, A.D.; Fontaine, G.; Bellayer, S.; Bourbigot, S. Salen based Schiff bases to flame retard thermoplastic polyurethane mimicking operational strategies of thermosetting resin. RSC Adv. 2015, 5, 48224–48235. [Google Scholar] [CrossRef]
- Punnakitikashem, P.; Truong, D.; Menon, J.U.; Nguyen, K.T.; Hong, Y. Electrospun biodegradable elastic polyurethane scaffolds with dipyridamole release for small diameter vascular grafts. Acta Biomater. 2014, 10, 4618–4628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, A.L. Vaginal mesh extrusion associated with use of Mentor transobturator sling. Urology 2005, 66, 995–999. [Google Scholar] [CrossRef]
- Genina, N.; Holländer, J.; Jukarainen, H.; Mäkilä, E.; Salonen, J.; Sandler, N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. Eur. J. Pharm. Sci. 2016, 90, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.H.; Weisman, J.A.; Jammalamadaka, U.; Tappa, K.; Alexander, J.S.; Griffen, F.D. Three-dimensional printing of bioactive hernia meshes: In vitro proof of principle. Surgery 2017, 161, 1479–1481. [Google Scholar] [CrossRef]
- Boyer, C.J.; Ballard, D.H.; Weisman, J.A.; Hurst, S.; McGee, D.J.; Mills, D.K.; Woerner, J.E.; Jammalamadaka, U.; Tappa, K.; Alexander, J.S. Three-Dimensional Printing Antimicrobial and Radiopaque Constructs. 3D Print. Addit. Manuf. 2018, 5, 29–36. [Google Scholar] [CrossRef] [PubMed]
- CDC. Types of Healthcare-Associated Infections. Healthcare-Associated Infections (HAIs). Available online: https://www.cdc.gov/HAI/infectionTypes.html (accessed on 10 March 2019).
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Khan, H.A.; Ahmad, A.; Mehboob, R. Nosocomial infections and their control strategies. Asian Pac. J. Trop. Biomed. 2015, 5, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Lausch, K.R.; Fuursted, K.; Larsen, C.S.; Storgaard, M. Colonisation with multi-resistant Enterobacteriaceae in hospitalised Danish patients with a history of recent travel: A cross-sectional study. Travel Med. Infect. Dis. 2013, 11, 320–323. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.H.; Jammalamadaka, U.; Tappa, K.; Weisman, J.A.; Boyer, C.J.; Alexander, J.S.; Woodard, P.K. 3D printing of surgical hernia meshes impregnated with contrast agents: In vitro proof of concept with imaging characteristics on computed tomography. 3D Print. Med. 2018, 4, 13. [Google Scholar] [CrossRef]
- Calero Castro, F.J.; Yuste, Y.; Pereira, S.; Garvín, M.D.; López García, M.Á.; Padillo, F.J.; Portilla, F. Proof of concept, design, and manufacture via 3-D printing of a mesh with bactericidal capacity: Behaviour in vitro and in vivo. J. Tissue Eng. Regen. Med. 2019, 13, 1955–1964. [Google Scholar] [CrossRef]
- Qamar, N.; Abbas, N.; Irfan, M.; Hussain, A.; Arshad, M.S.; Latif, S.; Mehmood, F.; Ghori, M.U. Personalized 3D printed ciprofloxacin impregnated meshes for the management of hernia. J. Drug Deliv. Sci. Technol. 2019, 53, 101164. [Google Scholar] [CrossRef]
- Statement by FDA Commissioner Scott Gottlieb, M.D., on FDA Ushering in New Era of 3D Printing of Medical Products; Provides Guidance to Manufacturers of Medical Devices. Available online: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-fda-ushering-new-era-3d-printing-medical-products (accessed on 15 March 2019).






| Formulations | TPU (g) | Castor Oil (μL) | LFX (g) |
|---|---|---|---|
| TPU | 30 | - | - |
| 0.25% LFX | 30 | 30 | 0.075 |
| 0.50% LFX | 30 | 30 | 0.15 |
| 1.00% LFX | 30 | 30 | 0.3 |
| LFX Content (%) | Elastic Limit (N) | Tensile Stiffness (N/mm) | Fracture Force (N) | Elongation at Break (mm) | |
|---|---|---|---|---|---|
| TPU | 0.00 | 1.2 ± 0.4 | 0.44 ± 0.12 | - | - |
| LFX 0.25% | 0.25 | 1.0 ± 0.2 | 0.32 ± 0.06 | - | - |
| LFX 0.50% | 0.50 | 1.1 ± 0.1 | 0.37 ± 0.04 | - | - |
| LFX 1.00% | 1.00 | 1.3 ± 0.2 | 0.45 ± 0.08 | - | - |
| PP | 0.00 | 6.5 ± 0.2 | 6.05 ± 0.83 | 15. 42 ± 0.66 | 129 ± 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Robles, J.; Mancinelli, C.; Mancuso, E.; García-Romero, I.; Gilmore, B.F.; Casettari, L.; Larrañeta, E.; Lamprou, D.A. 3D Printing of Drug-Loaded Thermoplastic Polyurethane Meshes: A Potential Material for Soft Tissue Reinforcement in Vaginal Surgery. Pharmaceutics 2020, 12, 63. https://doi.org/10.3390/pharmaceutics12010063
Domínguez-Robles J, Mancinelli C, Mancuso E, García-Romero I, Gilmore BF, Casettari L, Larrañeta E, Lamprou DA. 3D Printing of Drug-Loaded Thermoplastic Polyurethane Meshes: A Potential Material for Soft Tissue Reinforcement in Vaginal Surgery. Pharmaceutics. 2020; 12(1):63. https://doi.org/10.3390/pharmaceutics12010063
Chicago/Turabian StyleDomínguez-Robles, Juan, Caterina Mancinelli, Elena Mancuso, Inmaculada García-Romero, Brendan F. Gilmore, Luca Casettari, Eneko Larrañeta, and Dimitrios A. Lamprou. 2020. "3D Printing of Drug-Loaded Thermoplastic Polyurethane Meshes: A Potential Material for Soft Tissue Reinforcement in Vaginal Surgery" Pharmaceutics 12, no. 1: 63. https://doi.org/10.3390/pharmaceutics12010063
APA StyleDomínguez-Robles, J., Mancinelli, C., Mancuso, E., García-Romero, I., Gilmore, B. F., Casettari, L., Larrañeta, E., & Lamprou, D. A. (2020). 3D Printing of Drug-Loaded Thermoplastic Polyurethane Meshes: A Potential Material for Soft Tissue Reinforcement in Vaginal Surgery. Pharmaceutics, 12(1), 63. https://doi.org/10.3390/pharmaceutics12010063