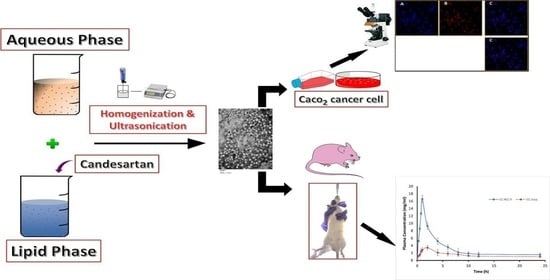
Enhancing the Oral Bioavailability of Candesartan Cilexetil Loaded Nanostructured Lipid Carriers: In Vitro Characterization and Absorption in Rats after Oral Administration
Abstract
:1. Introduction
- (1)
- The mucoadhesion properties of NLCs to the intestinal wall;
- (2)
- Emulsifying properties of surfactants used in formulation improve the permeability and solubility of the drug through the gastrointestinal tract membrane;
- (3)
- The nanosize of NLCs contributes to the enterocyte’s surface to increase. Consequently, improve and enhance drug permeability across the intestinal membrane [27,28]. Meanwhile, the nanometric size of NLCs adhere to the gastrointestinal tract easily for a long period and to intervillar spaces lead to the mean residence time to increase associated with increased bioavailability of the loaded drug;
- (4)
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of NLC
Fabrication of Nanostructured Lipid Carrier (NLC)
Fabrication of Fluorescent Nile Red Loaded Nanostructured Lipid Carriers (NR-NLCs)
2.2.2. Physicochemical Characterization of CC-NLC
Particle Size and Polydispersity Index
Zeta Potential Analysis
Encapsulation Efficiency (EE)
Determination of the Degree of Crystallinity and Polymorphism
Fourier Transform Infrared Spectroscopy (FT-IR)
Particle Morphology
2.2.3. Stability Study of CC-NLC
2.2.4. In Vitro Drug Release Study
2.2.5. In Vivo Studies
Absorption of CC Loaded NLC in the Gastrointestinal (GI) Tract
Uptake of NLCs in Caco-2 Cell Monolayer
Pharmacokinetic Behavior in Rats
2.2.6. Statistics
3. Results and Discussion
3.1. Fabrication of Candesartan Cilexetil Nanostructured Lipid Carrier (CC-NLC)
3.2. Physicochemical Characterization of CC-NLC Formulation
3.2.1. Particle Size, PDI, Zeta Potential, and Encapsulation Efficiency Measurements
3.2.2. Drug-Excipient Compatibility Studies (By DSC)
3.2.3. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
3.2.4. Morphology of CC-NLC
3.3. Storage Stability Study of CC-NLC
3.4. In Vitro Release Study
3.5. In Vivo Studies
3.5.1. Absorption of CC-NLC in the Gastrointestinal (GI) Tract
3.5.2. Cellular Uptake of Fluorescent Blank-NLC
3.5.3. Pharmacokinetic Behavior of CC-NLC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferreirós, N.; Dresen, S.; Alonso, R.M.; Weinmann, W. Hydrolysis and transesterification reactions of candesartan cilexetil observed during the solid phase extraction procedure. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 855, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Gurunath, S.; Nanjwade, B.K.; Patila, P.A. Enhanced solubility and intestinal absorption of candesartan cilexetil solid dispersions using everted rat intestinal sacs. Saudi Pharm. J. 2014, 22, 246–257. [Google Scholar] [CrossRef] [Green Version]
- Israili, Z.H. Clinical pharmacokinetics of angiotensin II (AT1) receptor blockers in hypertension. J. Hum. Hypertens. 2000, 14, S73–S86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurunath, S.; Nanjwade, B.K.; Patil, P.A. Oral bioavailability and intestinal absorption of candesartan cilexetil: Role of naringin as P-glycoprotein inhibitor. Drug Dev. Ind. Pharm. 2015, 41, 170–176. [Google Scholar] [CrossRef] [PubMed]
- AboulFotouh, K.; Allam, A.A.; El-Badry, M.; El-Sayed, A.M. Development and in vitro/in vivo performance of self-nanoemulsifying drug delivery systems loaded with candesartan cilexetil. Eur. J. Pharm. Sci. 2017, 109, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Dudhipala, N.; Veerabrahma, K. Candesartan cilexetil loaded solid lipid nanoparticles for oral delivery: Characterization, pharmacokinetic and pharmacodynamic evaluation. Drug Deliv. 2016, 23, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakkar, H.; Desai, J.; Parmar, M. Application of Box-Behnken design for optimization of formulation parameters for nanostructured lipid carriers of candesartan cilexetil. Asian J. Pharm. 2014, 8, 81. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, F.; Bu, H.; Xiao, J.; Li, Y. Solid lipid nanoparticles loading candesartan cilexetil enhance oral bioavailability: In vitro characteristics and absorption mechanism in rats. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 740–747. [Google Scholar] [CrossRef]
- Nekkanti, V.; Pillai, R.; Venkateshwarlu, V.; Harisudhan, T. Development and characterization of solid oral dosage form incorporating candesartan nanoparticles solid oral dosage form incorporating drug nanoparticles. Pharm. Dev. Technol. 2009, 14, 290–298. [Google Scholar] [CrossRef]
- Geçer, A.; Yıldız, N.; Çalımlı, A.; Turan, B. Trimethyl chitosan nanoparticles enhances dissolution of the poorly water soluble drug candesartan-cilexetil. Macromol. Res. 2010, 18, 986–991. [Google Scholar]
- Vaculikova, E.; Grunwaldova, V.; Kral, V.; Dohnal, J.; Jampilek, J. Preparation of candesartan and atorvastatin nanoparticles by solvent evaporation. Molecules 2012, 17, 13221–13234. [Google Scholar] [CrossRef]
- Sezgin-Bayindir, Z.; Antep, M.N.; Yuksel, N. Development and Characterization of Mixed Niosomes for Oral Delivery Using Candesartan Cilexetil as a Model Poorly Water-Soluble Drug. AAPS PharmSciTech 2014, 16, 108–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Zhang, Z.; Bu, H.; Huang, Y.; Gao, Z.; Shen, J.; Zhao, C.; Li, Y. Nanoemulsion improves the oral absorption of candesartan cilexetil in rats: Performance and mechanism. J. Control Release 2011, 149, 168–174. [Google Scholar] [CrossRef]
- Mahesh, R.D.; Umang, K.G.; Dhaval, D.M.; Kalpesh, A.P.; Ravi, M.; Seth, N.R. Formulation, optimization and characterization of candesartan cilexetil nanosuspension for in vitro dissolution enhancement. Afr. J. Pharm. Pharm. 2015, 9, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Nekkanti, V.; Karatgi, P.; Prabhu, R.; Pillai, R. Solid self-microemulsifying formulation for candesartan cilexetil. AAPS PharmSciTech 2010, 11, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravi, P.R.; Aditya, N.; Kathuria, H.; Malekar, S.; Vats, R. Lipid nanoparticles for oral delivery of raloxifene: Optimization, stability, in vivo evaluation and uptake mechanism. Eur. J. Pharm. Biopharm. 2014, 87, 114–124. [Google Scholar] [CrossRef]
- Paliwal, R.; Rai, S.; Vaidya, B.; Khatri, K.; Goyal, A.K.; Mishra, N.; Mehta, A.; Vyas, S.P. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 184–191. [Google Scholar] [CrossRef]
- Mathur, P.; Sharma, S.; Rawal, S.; Patel, B.; Patel, M.M. Fabrication, optimization, and in vitro evaluation of docetaxel-loaded nanostructured lipid carriers for improved anticancer activity. J. Liposome Res. 2020, 30, 182–196. [Google Scholar] [CrossRef]
- Attama, A.A.; Momoh, M.A.; Builders, P.F. Lipid nanoparticulate drug delivery systems: A revolution in dosage form design and development. In Recent Advances in Novel Drug Carrier Systems; InTech Publisher: Rijeka, Croatia, 2012; pp. 107–140. [Google Scholar]
- Poonia, N.; Kharb, R.; Lather, V.; Pandita, D. Nanostructured lipid carriers: Versatile oral delivery vehicle. Future Sci. OA 2016, 2, FSO135. [Google Scholar] [CrossRef] [Green Version]
- Mendes, I.T.; Ruela, A.L.M.; Carvalho, F.C.; Freitas, J.T.J.; Bonfilio, R.; Pereira, G.R. Development and characterization of nanostructured lipid carrier-based gels for the transdermal delivery of donepezil. Colloids Surf. B Biointerfaces 2019, 177, 274–281. [Google Scholar] [CrossRef]
- Rawal, S.; Patel, M.M. Threatening cancer with nanoparticle aided combination oncotherapy. J. Control. Release 2019, 301, 76–109. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Andreani, T.; Macedo, A.S.; Fangueiro, J.F.; Santana, M.H.A.; Silva, A.M.; Souto, E.B. Current state-of-art and new trends on lipid nanoparticles (SLN and NLC) for oral drug delivery. J. Drug Deliv. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.U.; Patel, M.M. Lipid Nanoparticulate Systems; Elsevier Inc.: Amsterdam, the Netherlands, 2018; ISBN 9780128136874. [Google Scholar]
- Wa Kasongo, K.; Shegokar, R.; Müller, R.H.; Walker, R.B. Formulation development and in vitro evaluation of didanosine-loaded nanostructured lipid carriers for the potential treatment of AIDS dementia complex. Drug Dev. Ind. Pharm. 2011, 37, 396–407. [Google Scholar] [CrossRef]
- Muchow, M.; Maincent, P.; Müller, R.H. Lipid nanoparticles with a solid matrix (SLN®, NLC®, LDC®) for oral drug delivery. Drug Dev. Ind. Pharm. 2008, 34, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, M.; Su, Z.; Sun, M.; Ping, Q. Size-exclusive effect of nanostructured lipid carriers on oral drug delivery. Int. J. Pharm. 2016, 511, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, R.N.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharm. 2004, 58, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Bargoni, A.; Cavalli, R.; Caputo, O.; Fundarò, A.; Maria, R.G.; Paolo, Z.G. Solid lipid nanoparticles in lymph and plasma after duodenal administration to rats. Pharm. Res. 1998, 15, 745–750. [Google Scholar] [CrossRef]
- Ros, E. Intestinal absorption of triglyceride and cholesterol. Dietary and pharmacological inhibition to reduce cardiovascular risk. Atherosclerosis 2000, 151, 357–379. [Google Scholar] [CrossRef]
- Kumar, V.V.; Chandrasekar, D.; Ramakrishna, S.; Kishan, V.; Rao, Y.M.; Diwan, P.V. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: Influence of wax and glyceride lipids on plasma pharmacokinetics. Int. J. Pharm. 2007, 335, 167–175. [Google Scholar] [CrossRef]
- Anwar, W.; Dawaba, H.M.; Afouna, M.I.; Samy, A.M. Screening study for formulation variables in preparation and characterization of candesartan cilexetil loaded nanostructured lipid carriers. Pharm. Res. 2019, 4, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Nnamani, P.O.; Hansen, S.; Windbergs, M.; Lehr, C.M. Development of artemether-loaded nanostructured lipid carrier (NLC) formulation for topical application. Int. J. Pharm. 2014, 477, 208–217. [Google Scholar] [CrossRef]
- Niamprem, P.; Srinivas, S.P.; Tiyaboonchai, W. Penetration of Nile red-loaded nanostructured lipid carriers (NLCs) across the porcine cornea. Colloids Surf. B Biointerfaces 2019, 176, 371–378. [Google Scholar] [CrossRef]
- Jiang, S.; Li, S.; Hu, J.; Xu, X.; Wang, X.; Kang, X.; Qi, J.; Lu, X.; Wu, J.; Du, Y.; et al. Combined delivery of angiopoietin-1 gene and simvastatin mediated by anti-intercellular adhesion molecule-1 antibody-conjugated ternary nanoparticles for acute lung injury therapy. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Kaithwas, V.; Dora, C.P.; Kushwah, V.; Jain, S. Nanostructured lipid carriers of olmesartan medoxomil with enhanced oral bioavailability. Colloids Surf. B Biointerfaces 2017, 154, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Tang, X.; Yang, M.; Yang, D.; Liu, J. Transdermal drug delivery of triptolide-loaded nanostructured lipid carriers: Preparation, pharmacokinetic and evaluation for rheumatoid arthritis. Int. J. Pharm 2019, 554, 235–244. [Google Scholar] [CrossRef]
- Wolf, M.; Reiter, F.; Heuser, T.; Kotisch, H.; Klang, V.; Valenta, C. Monoacyl-phospatidylcholine based drug delivery systems for lipophilic drugs: Nanostructured lipid carriers vs. nano-sized emulsions. J. Drug Deliv. Sci. Technol. 2018, 46, 490–497. [Google Scholar] [CrossRef]
- Marques, A.C.; Rocha, A.I.; Leal, P.; Estanqueiro, M.; Lobo, J.M.S. Development and characterization of mucoadhesive buccal gels containing lipid nanoparticles of ibuprofen. Int. J. Pharm. 2017, 533, 455–462. [Google Scholar] [CrossRef]
- Tatke, A.; Dudhipala, N.; Janga, K.Y.; Balguri, S.P.; Avula, B.; Jablonski, M.M.; Majumdar, S. In situ gel of triamcinolone acetonide-loaded solid lipid nanoparticles for improved topical ocular delivery: Tear kinetics and ocular disposition studies. Nanomaterials 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Saedi, A.; Rostamizadeh, K.; Parsa, M.; Dalali, N.; Ahmadi, N. Preparation and characterization of nanostructured lipid carriers as drug delivery system: Influence of liquid lipid types on loading and cytotoxicity. Chem. Phys. Lipids 2018, 216, 65–72. [Google Scholar] [CrossRef]
- Pornputtapitak, W.; Pantakitcharoenkul, J.; Teeranachaideekul, V.; Sinthiptharakoon, K.; Sapcharoenkun, C.; Meemuk, B. Effect of oil content on physiochemical characteristics of γ-Oryzanol-loaded nanostructured lipid carriers. J. Oleo Sci. 2019, 68, 699–707. [Google Scholar] [CrossRef] [Green Version]
- Seyed Yagoubi, A.; Shahidi, F.; Mohebbi, M.; Varidi, M.; Golmohammadzadeh, S. Preparation, characterization and evaluation of physicochemical properties of phycocyanin-loaded solid lipid nanoparticles and nanostructured lipid carriers. J. Food Meas. Charact. 2018, 12, 378–385. [Google Scholar] [CrossRef]
- Kanwar, R.; Gradzielski, M.; Prevost, S.; Kaur, G.; Clemens, D.; Appavou, M.S.; Mehta, S.K. Effect of lipid chain length on nanostructured lipid carriers: Comprehensive structural evaluation by scattering techniques. J. Colloid Interface Sci. 2019, 534, 95–104. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Sbaraglini, M.L.; Talevi, A.; Couyoupetrou, M.; Di Ianni, M.; Pesce, G.O.; Alvarez, V.A.; Bruno-Blanch, L.E.; Castro, G.R.; Ruiz, M.E.; et al. Carbamazepine-loaded solid lipid nanoparticles and nanostructured lipid carriers: Physicochemical characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2018, 167, 73–81. [Google Scholar] [CrossRef]
- Gruberová, L.; Kratochvil, B. Biorelevant dissolution of candesartan cilexetil. ADMET DMPK 2017, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Li, H.L.; Zhao, X.B.; Ma, Y.K.; Zhai, G.X.; Li, L.B.; Lou, H.X. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control Release 2009, 133, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.V.; Darling, I.M.; Morris, M.E. Choline uptake in human intestinal Caco-2 cells is carrier-mediated. J. Nutr. 2018, 133, 2607–2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietzonka, P.; Rothen-Rutishauser, B. Transfer of lipophilic markers from PLGA and polystyrene nanoparticles to caco-2 monolayers mimics particle uptake. Pharm. Res. 2002, 19, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Maestrini, L.; Maestrelli, F.; Mennini, N.; Mura, P.; Ghelardini, C.; Mannelli, L.D.C. Design, characterization and in vivo evaluation of nanostructured lipid carriers (NLC) as a new drug delivery system for hydrochlorothiazide oral administration in pediatric therapy. Drug Deliv. 2018, 25, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef]
- Elbahwy, I.A.; Ibrahim, H.M.; Ismael, H.R.; Kasem, A.A. Enhancing bioavailability and controlling the release of glibenclamide from optimized solid lipid nanoparticles. J. Drug Deliv. Sci. Technol. 2017, 38, 78–89. [Google Scholar] [CrossRef]
- Burra, M.; Jukanti, R.; Janga, K.Y.; Sunkavalli, S.; Velpula, A.; Ampati, S.; Jayaveera, K.N. Enhanced intestinal absorption and bioavailability of raloxifene hydrochloride via lyophilized solid lipid nanoparticles. Adv. Powder Technol. 2013, 24, 393–402. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Amin, S.; Ahmad, J. Improved pharmacokinetics and antihyperlipidemic efficacy of rosuvastatin-loaded nanostructured lipid carriers. J. Drug Target. 2017, 25, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, H.; Zhou, K.; Wang, L.; Dong, S.; Wang, D.; Chen, W. Nanostructured lipid carriers as a delivery system of biochanin A. Drug Deliv. 2013, 20, 331–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control Release 2008, 127, 97–109. [Google Scholar] [CrossRef]
- Noh, G.Y.; Suh, J.Y.; Park, S.N. Ceramide-based nanostructured lipid carriers for transdermal delivery of isoliquiritigenin: Development, physicochemical characterization, and in vitro skin permeation studies. Korean J. Chem. Eng. 2017, 34, 400–406. [Google Scholar] [CrossRef]
- López-García, R.; Ganem-Rondero, A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Occlusive effect and penetration enhancement ability. J. Cosmet. Dermatol. Sci. Appl. 2015, 05, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Teeranachaideekul, V.; Boonme, P.; Souto, E.B.; Müller, R.H.; Junyaprasert, V.B. Influence of oil content on physicochemical properties and skin distribution of Nile red-loaded NLC. J. Control Release 2008, 128, 134–141. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Souto, E.B.; Junyaprasert, V.B.; Müller, R.H. Cetyl palmitate-based NLC for topical delivery of Coenzyme Q10—Development, physicochemical characterization and in vitro release studies. Eur. J. Pharm. Biopharm. 2007, 67, 141–148. [Google Scholar] [CrossRef]
- Unruh, T.; Bunjes, H.; Westesen, K.; Koch, M.H.J. Investigations on the melting behaviour of triglyceride nanoparticles. Colloid Polym. Sci. 2001, 279, 398–403. [Google Scholar] [CrossRef]
- Abdelbary, G.; Fahmy, R.H. Diazepam-Loaded solid lipid nanoparticles: Design and characterization. AAPS PharmSciTech 2009, 10, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Cavalli, R.; Caputo, O.; Carlotti, M.E.; Trotta, M.; Scarnecchia, C.; Gasco, M.R. Sterilization and freeze-drying of drug-free and drug-loaded solid lipid nanoparticles. Int. J. Pharm. 1997, 148, 47–54. [Google Scholar] [CrossRef]
- Gardouh, A. Design and characterization of glyceryl monostearate solid lipid nanoparticles prepared by high shear homogenization. Br. J. Pharm. Res. 2013, 3, 326–346. [Google Scholar] [CrossRef]
- Dingler, A.; Blum, R.P.; Niehus, H.; Müller, R.H.; Gohla, S. Solid lipid nanoparticles (SLN(TM)/Lipopearls(TM))—A pharmaceutical and cosmetic carrier for the application of vitamin E in dermal products. J. Microencapsul. 1999, 16, 751–767. [Google Scholar] [CrossRef]
- Westesen, K.; Siekmann, B.; Koch, M.H.J. Investigations on the physical state of lipid nanoparticles by synchrotron radiation X-ray diffraction. Int. J. Pharm. 1993, 93, 189–199. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2010, 12, 62–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppe, K.; Sznitowska, M. The effect of polysorbate 20 on solubility and stability of candesartan cilexetil in dissolution media. AAPS PharmSciTech 2014, 15, 1116–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safwat, S.; Ishak, R.A.H.; Hathout, R.M.; Mortada, N.D. Nanostructured lipid carriers loaded with simvastatin: Effect of PEG/glycerides on characterization, stability, cellular uptake efficiency and in vitro cytotoxicity. Drug Dev. Ind. Pharm. 2017, 43, 1112–1125. [Google Scholar] [CrossRef]
- Mcclellan, K.J.; Goa, K.L.; Wales, N.S.; Dahlöf, B. Candesartan Cilexetil. Adis Drug Eval. 1998, 56, 847–869. [Google Scholar] [CrossRef]
- Shimizu, M.; Wang, Q.D.; Sjöquist, P.O.; Rydén, L. The angiotensin II AT1 receptor antagonist candesartan at antihypertensive plasma concentrations reduces damage induced by ischemia-reperfusion. Cardiovasc. Drugs 1999, 13, 347–353. [Google Scholar] [CrossRef]
- Dahan, A.; Hoffman, A. Rationalizing the selection of oral lipid based drug delivery systems by an in vitro dynamic lipolysis model for improved oral bioavailability of poorly water soluble drugs. J. Control Release 2008, 129, 1–10. [Google Scholar] [CrossRef]








| Formula Composition | |
|---|---|
| Lipid Phase | |
| Solid lipids 70% of lipids | GMS |
| Liquid lipids 30% of lipids | Capryol™ 90 |
| Drug = 5% of total lipids (w/w) | CC |
| Aqueous Phase | |
| Surfactants 2.5% (w/w) by ratio (1:1) | Lutrol® F127: Cremophor® RH |
| Water | Water |
| Physicochemical Characterization | |
| Particle size (PZ) | 121.6 ± 6.2 nm |
| Poly dispersity index (PDI) | 0.26 ± 0.03 |
| Zeta potential (ZP) | −26.5 ± 2.9 mV |
| Encapsulation efficiency (EE) | 96.23 ± 3.14% |
| Storage Condition | Size ± SD (nm) | PDI ± SD | Zeta ± SD (mV) | EE ± SD% | Visual Observation |
|---|---|---|---|---|---|
| Fresh | 121.6 ± 6.20 | 0.26 ± 0.03 | −26.5 ± 2.90 | 96.23 ± 3.14 | Clear emulsion |
| Refrigerator | |||||
| 1 month | 121.8 ± 7.23 | 0.28 ± 0.02 | −26.1 ± 2.05 | 95.86 ± 3.91 | Clear emulsion |
| 2 months | 122.1 ± 4.44 | 0.30 ± 0.05 | −25.91 ± 3.02 | 95.38 ± 4.71 | Clear emulsion |
| 3 months | 122.8 ± 5.25 NS | 0.31 ± 0.03 NS | −25.73 ± 0.58 NS | 94.91 ± 3.71 NS | Clear emulsion |
| Room Temperature (25 °C, RH 65%) | |||||
| 1 month | 122.5 ± 6.23 | 0.33 ± 0.03 | −25.2 ± 4.45 | 94.84 ± 5.91 | Clear emulsion |
| 2 months | 123.7 ± 8.13 | 0.39 ± 0.02 | −24.3 ± 5.23 | 94.10 ± 6.75 | Clear emulsion |
| 3 months | 124.6 ± 4.15 NS | 0.43 ± 0.02 NS | −23.52 ± 0.51 NS | 93.92 ± 4.59 NS | Clear emulsion |
| Parameters | Oral Administration of CC-NLC (10 mg/kg) Stomach | Oral Administration of CC-NLC (10 mg/kg) Intestine |
|---|---|---|
| AUC 0–end (µg h/mL) | 15.56 ± 5.65 | 35.87 ± 4.24 * |
| AUC 0–t (µg h/mL) | 3.31 ± 0.72 | 21.69 ± 1.52 *** |
| Cmax (µg/mL) | 2.12 ± 1.20 | 16.6 ± 2.40 *** |
| tmax (h) | 0.75 ± 0.12 | 1.00 ± 0.14 |
| t½ (h) | 5.02 ± 1.02 | 1.05 ± 1.03 * |
| MRT (h) | 1.80 ± 0.26 | 1.46 ± 0.68 |
| Frela (%) | - | 655.28 |
| Parameters | Oral Administration of CC Suspension (10 mg/kg) | Oral Administration of CC-NLC9 (10 mg/kg) |
|---|---|---|
| AUC 0–end (µg h/mL) | 64.35 ± 3.20 | 98.53 ± 4.20 *** |
| AUC 0–t (µg h/mL) | 35.70 ± 2.10 | 77.52 ± 3.40 *** |
| Cmax (µg/mL) | 3.44 ± 1.20 | 16.58 ± 2.40 ** |
| tmax (h) | 2.00 ± 0.14 | 1.00 ± 0.12 ** |
| t½ (h) | 9.51 ± 1.15 | 19.65 ± 2.18 |
| MRT (h) | 10.79 ± 3.10 | 15.99 ± 4.20 |
| Frela | - | 217.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwar, W.; Dawaba, H.M.; Afouna, M.I.; Samy, A.M.; Rashed, M.H.; Abdelaziz, A.E. Enhancing the Oral Bioavailability of Candesartan Cilexetil Loaded Nanostructured Lipid Carriers: In Vitro Characterization and Absorption in Rats after Oral Administration. Pharmaceutics 2020, 12, 1047. https://doi.org/10.3390/pharmaceutics12111047
Anwar W, Dawaba HM, Afouna MI, Samy AM, Rashed MH, Abdelaziz AE. Enhancing the Oral Bioavailability of Candesartan Cilexetil Loaded Nanostructured Lipid Carriers: In Vitro Characterization and Absorption in Rats after Oral Administration. Pharmaceutics. 2020; 12(11):1047. https://doi.org/10.3390/pharmaceutics12111047
Chicago/Turabian StyleAnwar, Walid, Hamdy M. Dawaba, Mohsen I. Afouna, Ahmed M. Samy, Mohammed H. Rashed, and Abdelaziz E. Abdelaziz. 2020. "Enhancing the Oral Bioavailability of Candesartan Cilexetil Loaded Nanostructured Lipid Carriers: In Vitro Characterization and Absorption in Rats after Oral Administration" Pharmaceutics 12, no. 11: 1047. https://doi.org/10.3390/pharmaceutics12111047
APA StyleAnwar, W., Dawaba, H. M., Afouna, M. I., Samy, A. M., Rashed, M. H., & Abdelaziz, A. E. (2020). Enhancing the Oral Bioavailability of Candesartan Cilexetil Loaded Nanostructured Lipid Carriers: In Vitro Characterization and Absorption in Rats after Oral Administration. Pharmaceutics, 12(11), 1047. https://doi.org/10.3390/pharmaceutics12111047