Printability of External and Internal Structures Based on Digital Light Processing 3D Printing Technique
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Printability Evaluation of External and Internal Structures
2.3. Mechanical Properties of the Printed Material
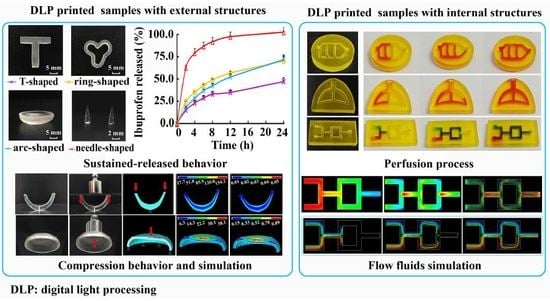
2.4. Preparation and Compression Behavior of Implants
2.5. In Vitro Dissolution of Implants
2.6. Preparation and Evaluation of Physiological Channels
3. Results and Discussion
3.1. The Effect of Photoabsorbers
3.2. Effect of PEGDA Concentration
3.3. Effect of Layer Height
3.4. Effect of Exposure Time
3.5. Compression Behavior and Simulation of Implants
3.6. In Vitro Dissolution of Implants
3.7. Perfusion Behavior and Simulation of Physiological Channels
4. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
Nomenclature
| DLP | digital light processing |
| PEGDA | polyethylene glycol diacrylate |
| DPPO | diphenyl(2,4,6-trimethylbenzoyl) phosphine oxide |
| DS | diclofenac sodium |
| Dmin | the minimum printable diameter |
| ΔL | inner arc length |
| ΔA | cavity area |
| Rf | cavity fusion rate |
| σb | tensile stress at break |
| εb | elongation at break |
| σv | Von Mises stress |
| εv | Von Mises strain |
| CFD | computational fluid dynamics |
| Rx-I | 100% PEGDA |
| Rx-II | 75% PEGDA with 0.25 mg/mL tartrazine |
| Rx-III | PEGDA: PEG300 = 80: 20 |
References
- Krkobabic, M.; Medarevic, D.; Cvijic, S.; Grujic, B.; Ibric, S. Hydrophilic excipients in digital light processing (DLP) printing of sustained release tablets: Impact on internal structure and drug dissolution rate. Int. J. Pharm. 2019, 572, 118790. [Google Scholar] [CrossRef]
- Lin, W.S.; Harris, B.T.; Pellerito, J.; Morton, D. Fabrication of an interim complete removable dental prosthesis with an in-office digital light processing three-dimensional printer: A proof-of-concept technique. J. Prosthet. Dent. 2018, 120, 331–334. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, Y.S.; Jung, H.D.; Hwang, C.J.; Baik, H.S.; Cha, J.Y. Precision and trueness of dental models manufactured with different 3-dimensional printing techniques. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.; Seo, Y.B.; Kim, D.Y.; Lee, J.S.; Lee, Y.J.; Lee, H.; Ajiteru, O.; Sultan, M.T.; Lee, O.J.; Kim, S.H.; et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 2019, 232, 119679. [Google Scholar] [CrossRef]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Eshel-Green, T.; Eliyahu, S.; Avidan-Shlomovich, S.; Bianco-Peled, H. PEGDA hydrogels as a replacement for animal tissues in mucoadhesion testing. Int. J. Pharm. 2016, 506, 25–34. [Google Scholar] [CrossRef]
- Burke, G.; Barron, V.; Geever, T.; Geever, L.; Devine, D.M.; Higginbotham, C.L. Evaluation of the materials properties, stability and cell response of a range of PEGDMA hydrogels for tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 99, 1–10. [Google Scholar] [CrossRef]
- Na, K.; Shin, S.; Lee, H.; Shin, D.; Baek, J.; Kwak, H.; Park, M.; Shin, J.; Hyun, J. Effect of solution viscosity on retardation of cell sedimentation in DLP 3D printing of gelatin methacrylate/silk fibroin bioink. J. Ind. Eng. Chem. 2018, 61, 340–347. [Google Scholar] [CrossRef]
- Mishra, R.; Roux, B.M.; Posukonis, M.; Bodamer, E.; Brey, E.M.; Fisher, J.P.; Dean, D. Effect of prevascularization on in vivo vascularization of poly(propylene fumarate)/fibrin scaffolds. Biomaterials 2016, 77, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, H.; Cuchiara, M.L.; Durst, C.A.; Cuchiara, M.P.; Lin, C.J.; West, J.L.; Grande-Allen, K.J. Fabrication and mechanical evaluation of anatomically-inspired quasilaminate hydrogel structures with layer-specific formulations. Ann. Biomed. Eng. 2013, 41, 398–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroli, B. Photopolymerization of biomaterials: Issues and potentialities in drug delivery, tissue engineering, and cell encapsulation applications. J. Chem. Technol. Biotechnol. 2006, 81, 491–499. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Ma, X.; Wang, H.; Ye, F. Influence of polymerization conditions on the refractive index of poly(ethylene glycol) diacrylate (PEGDA) hydrogels. Appl. Phys. A Mater. Sci. Process. 2018, 124. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef]
- Guerra, A.J.; Lammel-Lindemann, J.; Katko, A.; Kleinfehn, A.; Rodriguez, C.A.; Catalani, L.H.; Becker, M.L.; Ciurana, J.; Dean, D. Optimization of photocrosslinkable resin components and 3D printing process parameters. Acta Biomater. 2019, 97, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Mitteramskogler, G.; Gmeiner, R.; Felzmann, R.; Gruber, S.; Hofstetter, C.; Stampfl, J.; Ebert, J.; Wachter, W.; Laubersheimer, J. Light curing strategies for lithography-based additive manufacturing of customized ceramics. Addit. Manuf. 2014, 1–4, 110–118. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, Z.; Zhang, Q.; Qi, H.J.; Fang, D. Mechanics of shape distortion of DLP 3D printed structures during UV post-curing. Soft Matter 2019, 15, 6151–6159. [Google Scholar] [CrossRef]
- Jin, T.; Stanciulescu, I. Numerical investigation of the influence of pattern topology on the mechanical behavior of PEGDA hydrogels. Acta Biomater. 2017, 49, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Robles, M.P.; Buanz, A.; Basit, A.W.; Gaisford, B.S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, L.; Yuan, F.; Fu, H.; Shan, W. Preparation of sustained release capsules by electrostatic dry powder coating, using traditional dip coating as reference. Int. J. Pharm. 2018, 543, 345–351. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, J.; Shen, L.; Shan, W. Simulation and evaluation of rupturable coated capsules by finite element method. Int. J. Pharm. 2017, 519, 220–229. [Google Scholar] [CrossRef]
- Morgan, S.R.; Pilia, N.; Hewitt, M.; Moses, R.L.; Moseley, R.; Lewis, P.N.; Morrison, P.W.J.; Kelly, S.L.; Parker, J.E.; Whitaker, D.; et al. Controlled In Vitro Delivery of Voriconazole and Diclofenac to the Corneausing Contact Lenses for the Treatment of Acanthamoeba Keratiti. Int. J. Pharm. 2020, 579, 119102. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Grinberg, V.Y.; Burova, T.V.; Concheiro, A. Stimuli-sensitive crosslinked hydrogels as drug delivery systems: Impact of the drug on the responsiveness. Int. J. Pharm. 2020, 579, 119157. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, C.; Xiao, X. Polydopamine modified TiO2 nanotube arrays as a local drug delivery system for ibuprofen. J. Drug Deliv. Sci. Technol. 2020, 56, 101537. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, N.; Zhou, Y.; Shan, W.; Shen, J. Mechanistic study on rapid fabrication of fibrous films via centrifugal melt spinning. Int. J. Pharm. 2019, 560, 155–165. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 2018, 115, 11–18. [Google Scholar] [CrossRef]













| T-Shaped | Ring-Shaped | U-Shaped | Arc-Shaped | Needle-Shaped | |
|---|---|---|---|---|---|
| surface area (m2) | 5.8 × 10−4 | 5.8 × 10−4 | 4.7 × 10−4 | 7.5 × 10−4 | 2.2 × 10−5 |
| volume (m3) | 4.2 × 10−7 | 4.2 × 10−7 | 4.6 × 10−7 | 5.8 × 10−7 | 3.6 × 10−9 |
| specific surface area (m2/m3) | 1.4 × 103 | 1.4 × 103 | 1.0 × 103 | 1.3 × 103 | 6.2 × 103 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhou, Y.; Lin, X.; Yang, Q.; Yang, G. Printability of External and Internal Structures Based on Digital Light Processing 3D Printing Technique. Pharmaceutics 2020, 12, 207. https://doi.org/10.3390/pharmaceutics12030207
Yang Y, Zhou Y, Lin X, Yang Q, Yang G. Printability of External and Internal Structures Based on Digital Light Processing 3D Printing Technique. Pharmaceutics. 2020; 12(3):207. https://doi.org/10.3390/pharmaceutics12030207
Chicago/Turabian StyleYang, Yan, Yanjun Zhou, Xiao Lin, Qingliang Yang, and Gengshen Yang. 2020. "Printability of External and Internal Structures Based on Digital Light Processing 3D Printing Technique" Pharmaceutics 12, no. 3: 207. https://doi.org/10.3390/pharmaceutics12030207
APA StyleYang, Y., Zhou, Y., Lin, X., Yang, Q., & Yang, G. (2020). Printability of External and Internal Structures Based on Digital Light Processing 3D Printing Technique. Pharmaceutics, 12(3), 207. https://doi.org/10.3390/pharmaceutics12030207