The Influence of Moisture Content and Temperature on the Long-Term Storage Stability of Freeze-Dried High Concentration Immunoglobulin G (IgG)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Formulation Characterisation
2.2. Modulated DSC
2.3. Case Study 1: Freeze Drying of a High Concentration IgG
2.4. Case Study 2: Optimum Moisture Content of IgG
2.5. Case Study 3: Comparison of Low and High Moisture FD Cycles
2.6. Diphtheria and Tetanus ELISA
2.7. SEC-HPLC
2.8. Residual Moisture Content
3. Results and Discussion
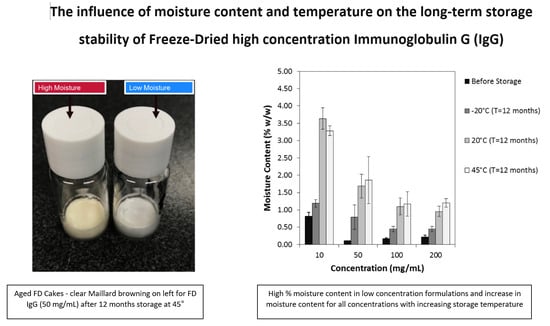
3.1. Case Study 1: Effect of Moisture Ingress and Excipient Ratio on Storage Stability of High Concentration IgG
3.2. Case Study 2: Optimum Moisture Content of IgG
3.3. Case Study 3: Stability of Induced “Low” Versus “High” Moisture FD Cycles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shire, S.J.; Shahrokh, Z.; Liu, J. Challenges in the development of high protein concentration formulations. J. Pharm. Sci. 2004, 93, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Warne, N.W. Development of high concentration protein biopharmaceuticals: The use of platform approaches in formulation development. Eur. J. Pharm. Biopharm. 2011, 78, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Garidel, P.; Presser, I. Lyophilization of High.-Concentration Protein Formulations. In Lyophilization of Pharmaceuticals and Biologicals: New Technologies and Approaches; Ward, K.R., Matejtschuk, P., Eds.; Springer Press: New York, NY, USA, 2019; pp. 291–325. [Google Scholar]
- Costantino, H.R. Excipients for Use in Lyophilized Pharmaceutical Peptides & Proteins. In Lyophilization of Biopharmaceuticals; Costantino, H.R., Pikal, M.J., Eds.; AAPS Press: Arlington, TX, USA, 2004; p. 139. [Google Scholar]
- Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000, 203, 1–60. [Google Scholar] [CrossRef]
- Beech, K.E.; Biddlecombe, J.G.; Van Der Walle, C.F.; Stevens, L.A.; Rigby, S.P.; Burley, J.C.; Allen, S. Insights into the influence of the cooling profile on the reconstitution times of amorphous lyophilized protein formulations. Eur. J. Pharm. Biopharm. 2015, 96, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Krishnan, S.; Ricci, M.S.; Shih, L.-Y.; Liu, D.; Gu, J.H.; Jameel, F. Rational design of lyophilized high concentration protein formulations-mitigating the challenge of slow reconstitution with multidisciplinary strategies. Eur. J. Pharm. Biopharm. 2013, 85, 287–293. [Google Scholar] [CrossRef]
- European Pharmacopoeia Commission. European Pharmacopoeia, 9th ed.; EDQM: Strasbourg, France, 2017. [Google Scholar]
- Wasserman, R.L. Gammaplex®5 and 10% in the treatment of primary immunodeficiency and chronic immune thrombocytopenic purpura. Immunotherapy 2017, 9, 1071–1088. [Google Scholar] [CrossRef]
- Chaigne, B.; Mouthon, L. Mechanisms of action of intravenous immunoglobulin. Transfus. Apher. Sci. 2017, 56, 45–49. [Google Scholar] [CrossRef]
- Breen, E.D.; Curley, J.G.; Overcashier, D.E.; Hsu, C.C.; Shire, S.J. Effect of moisture on the stability of a lyophilized humanized monoclonal antibody formulation. Pharm. Res. 2001, 18, 1345–1353. [Google Scholar] [CrossRef]
- Hsu, C.C.; Ward, C.A.; Pearlman, R.; Nguyen, H.M.; Yeung, D.A.; Curley, J.G. Determining the optimum residual moisture in lyophilized protein pharmaceuticals. Dev. Boil. Stand. 1992, 74, 255–270. [Google Scholar]
- Greiff, D. Protein structure and freeze-drying: The effects of residual moisture and gases. Cryobiol. 1971, 8, 145–152. [Google Scholar] [CrossRef]
- Pikal, M.J.; Shah, S. Moisture transfer from stopper to product and resulting stability implications. Dev. Boil. Stand. 1992, 74, 165–177. [Google Scholar]
- Haider, S.; Ashok, A. ICH Harmonised Tripartite Guideline. In Biotechnology: A Comprehensive Training Guide for the Biotechnology industry; CRC Press Inc.: Boca Raton, FL, USA, 2009; pp. 541–555. [Google Scholar]
- Tamizi, E.; Jouyban, A. Forced degradation studies of biopharmaceuticals: Selection of stress conditions. Eur. J. Pharm. Biopharm. 2016, 98, 26–46. [Google Scholar] [CrossRef] [PubMed]
- Halley, J.; Chou, Y.R.; Cicchino, C.; Huang, M.; Sharma, V.; Tan, N.C.; Thakkar, S.; Zhou, L.L.; Al-Azzam, W.; Cornen, S.; et al. An Industry Perspective on Forced Degradation Studies of Biopharmaceuticals: Survey Outcome and Recommendations. J. Pharm. Sci. 2020, 109, 6–21. [Google Scholar] [CrossRef] [Green Version]
- Van Hoeven, K.H.; Dale, C.; Foster, P.; Body, B. Comparison of Three Enzyme-Linked Immunosorbent Assays for Detection of Immunoglobulin G Antibodies to Tetanus Toxoid with Reference Standards and the Impact on Clinical Practice. Clin. Vaccine Immunol. 2008, 15, 1751–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, P.; Koza, S.; Bouvier, E.S.P. A Review Size Exclusion Chromatography for the Analysis of Protein Biotherapeutics and their Aggregates. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 2923–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tankersley, D.L.; Preston, M.; Finlayson, J. Immunoglobulin G dimer: An idiotype-anti-idiotype complex. Mol. Immunol. 1988, 25, 41–48. [Google Scholar] [CrossRef]
- Ford, A.W.; Dawson, P.J. Effect of Type of Container, Storage Temperature and Humidity on the Biological Activity of Freeze-dried Alkaline Phosphatase. Biologicals 1994, 22, 191–197. [Google Scholar] [CrossRef]
- Duralliu, A.; Matejtschuk, P.; Williams, D.R. Measuring the specific surface area (SSA) of freeze-dried biologics using inverse gas chromatography. Eur. J. Pharm. Biopharm. 2019, 142, 216–221. [Google Scholar] [CrossRef]
- Franks, F. Long–Term Stabilization of Biologicals. BioTechnology 1994, 12, 253–256. [Google Scholar] [CrossRef]
- Her, L.; Nail, S.L. Measurement of Glass Transition Temperatures of Freeze-Concentrated Solutes by Differential Scanning Calorimetry. Pharm. Res. 1994, 11, 54–59. [Google Scholar] [CrossRef]
- Shamblin, S.L.; Zografi, G. The effects of absorbed water on the properties of amorphous mixtures containing sucrose. Pharm. Res. 1999, 16, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Garidel, P.; Pevestorf, B.; Bahrenburg, S. Stability of buffer-free freeze-dried formulations: A feasibility study of a monoclonal antibody at high protein concentrations. Eur. J. Pharm. Biopharm. 2015, 97, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Garidel, P.; Kuhn, A.B.; Schäfer, L.V.; Karow-Zwick, A.R.; Blech, M. High-concentration proteins—How high is high? Eur. J. Pharm. Biopharm. 2017, 119, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Cicerone, M.T.; Pikal, M.J.; Qian, K.K. Stabilization of proteins in solid form. Adv. Drug Deliv. Rev. 2015, 93, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.; Shepherd, D.; Sun, J.; Tang, X.; Pikal, M.J. Effect of sorbitol and residual moisture on the stability of lyophilized antibodies: Implications for the mechanism of protein stabilization in the solid state. J. Pharm. Sci. 2005, 94, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Matejtschuk, P.; Rafiq, S.; Johnes, S.; Das, R.G. A comparison of vials with ampoules for the storage of biological reference materials. Biologicals 2005, 33, 63–70. [Google Scholar] [CrossRef]
- Duralliu, A.; Matejtschuk, P.; Dubey, S.; Koroma, H.; Gubinelli, F.; Williams, D.R. The influence of the closure format on the storage stability and moisture content of freeze-dried influenza antigen. Vaccine 2019, 37, 4485–4490. [Google Scholar] [CrossRef]
- Ferguson, M.; Wilkinson, D.E.; Heath, A.; Matejtschuk, P. The first international standard for antibodies to HPV 16. Vaccine 2011, 29, 6520–6526. [Google Scholar] [CrossRef]
- Wang, D. Formulation characterization, in Freeze Drying. In Lyophilization of Pharmaceutical and Biological Products, 3rd ed.; Rey, L., May, J.C., Eds.; Informa Healthcare: New York, NY, USA, 2010; pp. 245–265. [Google Scholar]
- Liu, W.R.; Langer, R.; Klibanov, A.M. Moisture-induced aggregation of lyophilized proteins in the solid state. Biotechnol. Bioeng. 1991, 37, 177–184. [Google Scholar] [CrossRef]
- Matejtschuk, P.; Phillips, P.; Andersen, M. Freeze-Drying of Biological Standards. In Lyophilization of Pharmaceutical and Biological Products, 2nd ed.; Rey, L., May, J.C., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 2004; pp. 317–352. [Google Scholar]
- Hubbard, A.; Bevan, S.; Matejtschuk, P. Impact of residual moisture and formulation on Factor VIII and Factor V recovery in lyophilized plasma reference materials. Anal. Bioanal. Chem. 2006, 387, 2503–2507. [Google Scholar] [CrossRef]
- Schüle, S.; Schulzfademrecht, T.; Garidel, P.; Bechtoldpeters, K.; Frieß, W. Stabilization of IgG1 in spray-dried powders for inhalation. Eur. J. Pharm. Biopharm. 2008, 69, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Kanojia, G.; Have, R.T.; Bakker, A.; Wagner, K.; Frijlink, H.W.; Kersten, G.F.A.; Amorij, J.-P. The Production of a Stable Infliximab Powder: The Evaluation of Spray and Freeze-Drying for Production. PLoS ONE 2016, 11, e0163109. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, G.; Vercellotti, J.R. Degradation of Sucrose, Glucose and Fructose in Concentrated Aqueous Solutions Under Constant pH Conditions at Elevated Temperature. J. Carbohydr. Chem. 2000, 19, 1305–1318. [Google Scholar] [CrossRef]
- Matejtschuk, P.; Harrison, P.; More, J.E. Dry Heat Treatment of Intravenous Immunoglobulin—Some Practical Considerations. Vox Sang. 1995, 68, 255–257. [Google Scholar] [CrossRef]
- Flink, J.M. Nonenzymatic Browning of Freeze-Dried Sucrose. J. Food Sci. 1983, 48, 539–542. [Google Scholar] [CrossRef]
- Patel, S.M.; Nail, S.L.; Pikal, M.J.; Geidobler, R.; Winter, G.; Hawe, A.; Davignino, J.; Gupta, S.R. Lyophilized drug product cake appearance: What is acceptable? J. Pharm Sci. 2017, 106, 1706–1721. [Google Scholar] [CrossRef]











| Cycle | Freezing Temperature (°C) | Freezing Ramp Rate (°C/min) | Freezing Hold Time (min) | Primary Drying Temperature (°C) | Primary Hold Time (min) | Secondary Drying Temperature (°C) | Secondary Drying Ramp Rate (°C/min) | Secondary Hold Time (min) |
|---|---|---|---|---|---|---|---|---|
| Low Moisture | −40 | 1.00 | 120 | −15 | 1200 | 30 | 0.15 | 600 |
| High Moisture | −40 | 1.00 | 120 | −40 | 900 | 15 | 0.18 | 60 |
| Storage Temperature (°C) | IgG Concentration (mg/mL) | Tg | Tg-T (°C) |
|---|---|---|---|
| −20 | 10 | 101 | 121 |
| −20 | 50 | 104 | 124 |
| −20 | 100 | 110 | 130 |
| −20 | 200 | 105 | 125 |
| 20 | 10 | 94 | 74 |
| 20 | 50 | 96 | 76 |
| 20 | 100 | 117 | 97 |
| 20 | 200 | 116 | 96 |
| 45 | 10 | 77 | 32 |
| 45 | 50 | 84 | 39 |
| 45 | 100 | 84 | 39 |
| 45 | 200 | 90 | 45 |
| Temperature (°C) | Initial Moisture Sample (% w/w) | Tg | T-Tg (°C) |
|---|---|---|---|
| −20 | 0.05 (A) | 115 | 135 |
| 0.67 (B) | 113 | 133 | |
| 3.02 (C) | 113 | 133 | |
| 4.95 (D) | 111 | 131 | |
| 20 | 0.05 (A) | 114 | 94 |
| 0.67 (B) | 111 | 91 | |
| 3.02 (C) | 111 | 91 | |
| 4.95 (D) | 110 | 90 | |
| 45 | 0.05 (A) | 112 | 67 |
| 0.67 (B) | 113 | 68 | |
| 3.02 (C) | 110 | 65 | |
| 4.95 (D) | 108 | 63 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duralliu, A.; Matejtschuk, P.; Stickings, P.; Hassall, L.; Tierney, R.; Williams, D.R. The Influence of Moisture Content and Temperature on the Long-Term Storage Stability of Freeze-Dried High Concentration Immunoglobulin G (IgG). Pharmaceutics 2020, 12, 303. https://doi.org/10.3390/pharmaceutics12040303
Duralliu A, Matejtschuk P, Stickings P, Hassall L, Tierney R, Williams DR. The Influence of Moisture Content and Temperature on the Long-Term Storage Stability of Freeze-Dried High Concentration Immunoglobulin G (IgG). Pharmaceutics. 2020; 12(4):303. https://doi.org/10.3390/pharmaceutics12040303
Chicago/Turabian StyleDuralliu, Arnold, Paul Matejtschuk, Paul Stickings, Laura Hassall, Robert Tierney, and Daryl R. Williams. 2020. "The Influence of Moisture Content and Temperature on the Long-Term Storage Stability of Freeze-Dried High Concentration Immunoglobulin G (IgG)" Pharmaceutics 12, no. 4: 303. https://doi.org/10.3390/pharmaceutics12040303
APA StyleDuralliu, A., Matejtschuk, P., Stickings, P., Hassall, L., Tierney, R., & Williams, D. R. (2020). The Influence of Moisture Content and Temperature on the Long-Term Storage Stability of Freeze-Dried High Concentration Immunoglobulin G (IgG). Pharmaceutics, 12(4), 303. https://doi.org/10.3390/pharmaceutics12040303