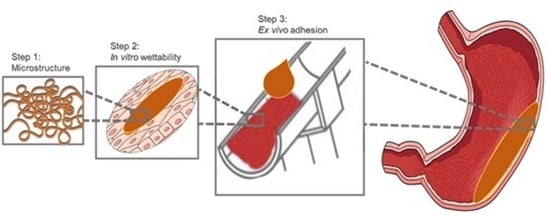
Investigations to Evaluate Gastric Mucoadhesion of an Organic Product to Ameliorate Gastritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Investigated Formulations
2.2. Macrorheological Measurements Taking into Account Interactions with Simulated Gastric Fluid and Gastric Mucins
2.3. Cryo-Scanning Electron Microscopy (Cryo-SEM) Preparation
2.4. Calculation of the Pore-Size Distribution
2.5. In Vitro Wettability Studies
2.5.1. AGS-Cell Cultivation
2.5.2. Contact Angle Measurements
2.6. Ex Vivo Studies with the Falling Lquid Film Technique
Ex Vivo Quantification with Franz Diffusion Cells
2.7. Statistical Analysis
3. Results
3.1. Macroscopical Evaluation of the Extracts
3.2. Rheological Analysis
3.3. Microstructure and Pore Size Distribution
3.4. In Vitro Wettability Studies
3.5. Ex Vivo Falling Liquid Studies
Ex Vivo Quantification via Franz Diffusion Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azer, S.A. Gastritis. In StatPe; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Vieth, M.; Neumann, H.; Falkeis, C. The diagnosis of gastritis. Diagnostic Histopathol. 2014, 20, 213–221. [Google Scholar] [CrossRef]
- Watari, J.; Chen, N.; Amenta, P.S.; Fukui, H.; Oshima, T.; Tomita, T.; Miwa, H.; Lim, K.J.; Das, K.M. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J. Gastroenterol. 2014, 20, 5461–5473. [Google Scholar] [CrossRef] [PubMed]
- Varbanova, M.; Frauenschläger, K.; Malfertheiner, P. Chronic gastritis—An update. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Zilli, A.; Elvevi, A.; Invernizzi, P. The changing face of chronic autoimmune atrophic gastritis: An updated comprehensive perspective. Autoimmun. Rev. 2019, 18, 215–222. [Google Scholar] [CrossRef]
- Toh, B.H. Diagnosis and classification of autoimmune gastritis. Autoimmun. Rev. 2014, 13, 459–462. [Google Scholar] [CrossRef]
- Waters, K.M.; Voltaggio, L. Gastritis: A pattern-based approach. Diagnostic Histopathol. 2017, 23, 513–520. [Google Scholar] [CrossRef]
- Assarzadegan, N.; Montgomery, E. Gastric polyps. Diagnostic Histopathol. 2017, 23, 521–529. [Google Scholar] [CrossRef]
- Cheung, L.Y. Pathogenesis, prophylaxis, and treatment of stress gastritis. Am. J. Surg. 1988, 156, 437–440. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Qin, L.L.; Song, Y.; Vidal-Alaball, J.; Liu, T.H. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst. Rev. 2018, 90412039. [Google Scholar] [CrossRef]
- Talebi Bezmin Abadi, A.; Yamaoka, Y. Helicobacter pylori therapy and clinical perspective. J. Glob. Antimicrob. Resist 2018, 14, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Asaka, M.; Kato, M.; Sakamoto, N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J. Gastroenterol. 2014, 49, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durham, R.M.; Shapiro, M.J. Stress gastritis revisited. Surg. Clin. N. Am. 1991, 71, 791–810. [Google Scholar] [CrossRef]
- Weiser, F.A.; Fangl, M.; Mosgoeller, W. Supplementation of Caricol®-Gastro reduces chronic gastritis disease associated pain. Neuroendocrinol. Lett. 2018, 39, 19–25. [Google Scholar] [PubMed]
- Müller, C.; Leithner, K.; Hauptstein, S.; Hintzen, F.; Salvenmoser, W.; Bernkop-Schnürch, A. Preparation and characterization of mucus-penetrating papain/poly(acrylic acid) nanoparticles for oral drug delivery applications. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef]
- Muss, C.; Mosgoeller, W.; Endler, T. Papaya preparation (Caricol®) in digestive disorders. Neuro Endocrinol. Lett. 2013, 34, 38–46. [Google Scholar]
- Osato, J.A.; Santiago, L.A.; Remo, G.M.; Cuadra, M.S.; Mori, A. Antimicrobial and antioxidant activities of unripe papaya. Life Sci. 1993, 53, 1383–1389. [Google Scholar] [CrossRef]
- Cho, C.H.; Han, P.W. Papain reduces gastric acid secretion induced by histamine and other secretagogues in anesthetized rats. Proc. Natl. Sci. Counc. Repub. China B 1984, 8, 177–181. [Google Scholar]
- Miller, S.S.; Fulcher, R.G. Microstructure and chemistry of the oat kernel. In Oats: Chemistry and Technology; American Association of Cereal Chemists, Inc. (AACC): Saint Paul, MN, USA, 2011; pp. 77–94. [Google Scholar]
- Yiu, S.H. Effects of processing and cooking on the structural and microchemical composition of oats. Food Struct. 1986, 5, 219–225. [Google Scholar]
- Hartunian Sowa, S.; White, P. Characterization of starch isolated from oat groats with different amounts of lipid. Cereal Chem. 1992, 69, 521–527. [Google Scholar]
- Choi, I.; Han, O.K.; Chun, J.; Kang, C.S.; Kim, K.H.; Kim, Y.K.; Cheong, Y.K.; Park, T.I.; Choi, J.S.; Kim, K.J. Hydration and pasting properties of oat (Avena sativa) flour. Prev. Nutr. Food Sci. 2012, 17, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Bailey, T.B.; White, P.J. Individual and Interactional Effects of β-Glucan, Starch, and Protein on Pasting Properties of Oat Flours. J. Agric. Food Chem. 2010, 58, 9198–9203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, Q.; Pu, Y.; Li, X.; Ma, Z.; Hu, X. Solvent retention capacities of oat flour. Int. J. Mol. Sci. 2017, 18, 590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug. Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.M.; Robinson, J.R.; Leung, S.H.S. Binding of acrylic polymers to mucin/epithelial surfaces: Structure-property relationships. Crit. Rev. Ther. Drug Carrier Syst. 1988, 5, 21–67. [Google Scholar] [PubMed]
- Mortazavi, S.A.; Smart, J.D. An investigation into the role of water movement and mucus gel dehydration in mucoadhesion. J. Control. Release 1993, 25, 197–203. [Google Scholar] [CrossRef]
- Cook, S.L.; Bull, S.P.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesion: A food perspective. Food Hydrocoll. 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Jabbari, E.; Wisniewski, N.; Peppas, N.A. Evidence of mucoadhesion by chain interpenetration at a poly (acrylic acid)/mucin interface using ATR-FTIR spectroscopy. J. Control. Release 1993, 26, 99–108. [Google Scholar] [CrossRef]
- Mikos, A.G.; Peppas, N.A. Measurement of the surface tension of mucin solutions. Int. J. Pharm. 1989, 53, 1–5. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 2005, 57, 1569–1582. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Aleinikova, I.N.; Toporov, Y.P. On the role of electrostatic forces in the adhesion of polymer particles to solid surfaces. Prog. Surf. Sci. 1994, 45, 119–123. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Toporov, Y.P.; Muller, V.M.; Aleinikova, I.N. On the relationship between the electrostatic and the molecular component of the adhesion of elastic particles to a solid surface. J. Colloid. Interface Sci. 1977, 58, 528–533. [Google Scholar] [CrossRef]
- Huang, Y.; Leobandung, W.; Foss, A.; Peppas, N.A. Molecular aspects of muco- and bioadhesion: Tethered structures and site-specific surfaces. J. Control. Release 2000, 65, 63–71. [Google Scholar] [CrossRef]
- Park, H.; Robinson, J.R. Mechanisms of Mucoadhesion of Poly/acrylic Acid) Hydrogels. Pharm. Res. 1987, 6, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Pund, S.; Joshi, A.; Vasu, K.; Nivsarkar, M.; Shishoo, C. Gastroretentive delivery of rifampicin: In vitro mucoadhesion and in vivo gamma scintigraphy. Int. J. Pharm. 2011, 411, 106–112. [Google Scholar] [CrossRef]
- Chen, H.; Rogalski, M.M.; Anker, J.N. Advances in functional X-ray imaging techniques and contrastagents. Phys. Chem. Chem. Phys. 2013, 14, 13469–13486. [Google Scholar] [CrossRef] [Green Version]
- Kita, M.; Negi, A.; Kawano, S.; Honda, Y.; Maegawa, S. Measurement of retinal adhesive force in the in vivo rabbit eye. Investig. Ophthalmol. Vis. Sci. 1990, 31, 624–628. [Google Scholar]
- Davidovich-Pinhas, M.; Bianco-Peled, H. Methods to Study Mucoadhesive Dosage Forms. Mucoadhesive Mater. Drug Deliv. Syst. 2014, 9781119941, 175–196. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Lubes, G.; Goodarzi, M. GC-MS based metabolomics used for the identification of cancer volatile organic compounds as biomarkers. J. Pharm. Biomed. Anal. 2017. [Google Scholar] [CrossRef]
- Esposito, P.; Colombo, I.; Lovrecich, M. Investigation of surface properties of some polymers by a thermodynamic and mechanical approach: Possibility of predicting mucoadhesion and biocompatibility. Biomaterials 1994, 15, 177–182. [Google Scholar] [CrossRef]
- Belgamwar, V.; Shah, V.; Surana, S.J. Formulation and evaluation of oral mucoadhesive multiparticulate system containing metoprolol tartarate: An in vitro-ex vivo characterization. Curr. Drug Deliv. 2009, 6, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Gradauer, K.; Vonach, C.; Leitinger, G.; Kolb, D.; Fröhlich, E.; Roblegg, E.; Bernkop-Schnürch, A.; Prassl, R. Chemical coupling of thiolated chitosan to preformed liposomes improves mucoadhesive properties. Int. J. Nanomed. 2012, 7, 2523–2534. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Vigani, B.; Bonferoni, M.C.; Sandri, G.; Caramella, C.; Ferrari, F. Rheological analysis and mucoadhesion: A 30 year-old and still active combination. J. Pharm. Biomed. Anal. 2018, 156, 232–238. [Google Scholar] [CrossRef]
- Tambe, A.; Mokashi, P.; Pandita, N. Ex-vivo intestinal absorption study of boswellic acid, cyclodextrin complexes and poloxamer solid dispersions using everted gut sac technique. J. Pharm. Biomed. Anal. 2019, 167, 66–73. [Google Scholar] [CrossRef]
- Tetyczka, C.; Griesbacher, M.; Absenger-Novak, M.; Fröhlich, E.; Roblegg, E. Development of nanostructured lipid carriers for intraoral delivery of Domperidone. Int. J. Pharm. 2017, 526, 188–198. [Google Scholar] [CrossRef]
- United States Pharmacopoeia; United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 1990.
- Van der Reijden, W.A.; van der Kwaak, H.; Vissink, A.; Veerman, E.C.; Amerongen, A.V.N. Treatment of xerostomia with polymer-based saliva substitutes in patients with Sjogren’s syndrome. Arthritis Rheum. 1996, 39, 57–63. [Google Scholar] [CrossRef]
- Collado-González, M.; González Espinosa, Y.; Goycoolea, F.M. Interaction Between Chitosan and Mucin: Fundamentals and Applications. Biomimetics 2019, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Bonferoni, M.C.; Ferrari, F.; Bertoni, M.; Caramella, C. Characterization of mucin interaction with three viscosity grades of sodium carboxymethylcellulose. Comparison between rheological and tensile testing. Eur. J. Pharm. Sci. 1996, 4, 189–196. [Google Scholar] [CrossRef]
- Rossi, S.; Bonferoni, M.C.; Lippoli, G.; Bertoni, M.; Ferrari, F.; Caramella, C.; Conte, U. Influence of mucin type on polymer-mucin rheological interactions. Biomaterials 1995, 16, 1073–1079. [Google Scholar] [CrossRef]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.; Tabor, R.F. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, B.; Bashiry, V. Shape analysis of a sessile drop on a flat solid surface. J. Adhes. 2019, 95, 929–942. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.Y.; Wirtz, D.; Hanes, J. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 2009, 61, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, F.; Eberth, K.; Smart, J.D. A rheological examination of the mucoadhesive/mucus interaction: The effect of mucoadhesive type and concentration. J. Control. Release 1998, 50, 167–178. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Wattanakorn, N.; Nunthanid, J.; Puttipipatkhachorn, S. Mucoadhesion of pectin as evidence by wettability and chain interpenetration. Carbohydr. Polym. 2008, 74, 458–467. [Google Scholar] [CrossRef]
- Starley, I.F.; Mohammed, P.; Schneider, G.; Bickler, S.W. The treatment of paediatric burns using topical papaya. Burns 1999, 25, 636–639. [Google Scholar] [CrossRef]
- Wang, M.; Wei, L.; Zhu, P.; Du, X.; Zhou, Y. Effect of inulin on rheological properties and in vitro digestion of potato starch paste. J. Chin. Cereals Oils Assoc. 2016, 31, 47–51. [Google Scholar]
- Westerlund, E.; Åman, P.; Andersson, R.; Andersson, R.E.; Rahman, S.M.M. Chemical characterization of water-soluble pectin in papaya fruit. Carbohydr. Polym. 1991, 15, 67–78. [Google Scholar] [CrossRef]
- Wu, P.; Dhital, S.; Williams, B.A.; Chen, X.D.; Gidley, M.J. Rheological and microstructural properties of porcine gastric digesta and diets containing pectin or mango powder. Carbohydr. Polym. 2016, 148, 216–226. [Google Scholar] [CrossRef] [Green Version]







| Caricol®-Gastro | Ingredients | Concentration (g) |
|---|---|---|
| 100 g aqueous gel | Papaya pulp (containing pectin, more specifically α-(1→4)-d-galacturonan main polymeric component) * Oat flour (containing starch and β-glucan as main polymeric component) * Apple juice concentrate * Natural flavoring | 39.44/100 g 6.25/100 g 10.5/100 g 0.08/100 g |
| Interaction Parameters (Differential) | Formula | Shear Rates |
|---|---|---|
| Δη/η | Δη = ηmixture * (ηformulation + ηmucin) | 0.1 s−1 (low), 5.18 s−1 (medium), 100 s−1 (high) |
| ΔG’/G’ | ΔG’ = G’mixture − (G’formulation + Gmucin) | |
| Δtan δ | Δtan δ = tan δmixture – (tan δformulation + tan δmucin) |
| Formulation | Contact Angle Horizontal | Contact Angle Inclined (35 °C) |
|---|---|---|
| Caricol®-Gastro mixed with 0.1 M HCl (1:1) | 38° ± 5° | 28° ± 1° ** |
| Oat I, II | Measurement not possible due to agglutination in the syringe and, consequently, no drop formation possible. | |
| MQ-water mixed with 0.1 M HCl (1:1) | 101° ± 2° | 70° ± 2° *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winter, C.; Hartl, S.; Kolb, D.; Leitinger, G.; Roblegg, E. Investigations to Evaluate Gastric Mucoadhesion of an Organic Product to Ameliorate Gastritis. Pharmaceutics 2020, 12, 331. https://doi.org/10.3390/pharmaceutics12040331
Winter C, Hartl S, Kolb D, Leitinger G, Roblegg E. Investigations to Evaluate Gastric Mucoadhesion of an Organic Product to Ameliorate Gastritis. Pharmaceutics. 2020; 12(4):331. https://doi.org/10.3390/pharmaceutics12040331
Chicago/Turabian StyleWinter, Christina, Sonja Hartl, Dagmar Kolb, Gerd Leitinger, and Eva Roblegg. 2020. "Investigations to Evaluate Gastric Mucoadhesion of an Organic Product to Ameliorate Gastritis" Pharmaceutics 12, no. 4: 331. https://doi.org/10.3390/pharmaceutics12040331
APA StyleWinter, C., Hartl, S., Kolb, D., Leitinger, G., & Roblegg, E. (2020). Investigations to Evaluate Gastric Mucoadhesion of an Organic Product to Ameliorate Gastritis. Pharmaceutics, 12(4), 331. https://doi.org/10.3390/pharmaceutics12040331