“Smart” Antimicrobial Nanocomplexes with Potential to Decrease Surgical Site Infections (SSI)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
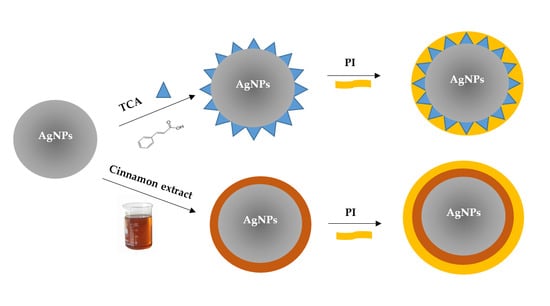
2.2. Preparation of Silver Nanoparticles with Trans-Cinnamic Acid (TCA) as Capping Agent (TCA-AgNP)
2.3. Preparation of Silver Nanoparticles with Cinnamon Bark Extract as Capping Agent (Cinn-AgNPs)
2.3.1. Preparation of Cinnamomum zeylanicum (Cinn) Bark Extract
2.3.2. Phytosynthesis of Nano-Scale Ag Particles (Cinn-AgNP)
2.4. Synthesis of Povidone Iodine (PI) Capped Silver Nanoparticles (TCA-AgNP-PI and Cinn-AgNP-PI)
2.5. Characterizations of Synthesized AgNP Samples
2.5.1. Morphological Examination and Particle-Size Measurement
2.5.2. Size and Zeta-Potential Analysis
2.5.3. UV-Vis Spectrophotometry
2.5.4. Fourier-Transform Infrared Spectroscopy
2.5.5. X-Ray Diffraction (XRD) Analysis of AgNPs
2.5.6. Characterization by Surface-Enhanced Raman Spectroscopy (SERS)
2.6. Bacterial Strains and Culturing
2.7. Investigation/Determination of Antibacterial and Antifungal Properties of TCA-AgNP, TCA-AgNP-PI, Cinn-AgNP, and Cinn-AgNP-PI
2.7.1. Procedure for Zone of Inhibition Plate Studies
2.7.2. Agar Well Diffusion Method
2.7.3. Disc Diffusion Method
2.8. Preparation of Sutures Coated with AgNPs Samples, Dip-Coating, and Characterization
2.9. Statistical Analysis
3. Results
3.1. Morphological Examination and Particle-Size Measurement
SEM and EDS Analysis
3.2. Particle Size, PDL, and Zeta Potential of TCA-AgNP, TCA-AgNP-PI, Cinn-AgNP, and Cinn-AgNP-PI
3.2.1. Dynamic Light Scattering (DLS)
3.2.2. Zeta Potential Analysis
3.3. UV-Vis Spectroscopic Analysis of TCA-AgNP, TCA-AgNP-PI, Cinn-AgNP, and Cinn-AgNP-PI
3.4. FT-IR Spectroscopic Analysis of TCA-AgNP, TCA-AgNP-PI, Cinn-AgNP, and Cinn-AgNP-PI
3.5. X-Ray Diffraction Measurement of TCA-AgNP, TCA-AgNP-PI, Cinn-AgNP, and Cinn-AgNP-PI
3.6. SERS Analysis of TCA-AgNP, TCA-AgNP-PI, Cinn-AgNP, and Cinn-AgNP-PI
3.7. Antimicrobial Activities of TCA-AgNP, TCA-AgNP-PI, Cinn-AgNP, and Cinn-AgNP-PI
3.7.1. Determination of Antimicrobial Properties of TCA-AgNP, TCA-AgNP-PI, Cinn-AgNP, and Cinn-AgNP-PI
3.7.2. Determination of Antimicrobial Properties of Sutures Dip-Coated with TCA-AgNP, TCA-AgNP-PI, Cinn-AgNP, and Cinn-AgNP-PI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shrivastava, S.; Shrivastava, P.; Ramasamy, J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 2018, 32, 76–77. [Google Scholar] [CrossRef]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539–563. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzzaman, N.; Pina, M.; Chivu, A.; Good, L. Polyhexamethylene biguanide and nadifloxacin self-assembled nanoparticles: Antimicrobial effects against intracellular methicillin-resistant Staphylococcus aureus. Polymers 2018, 10, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Tang, J.; Khare, T.; Kumar, V. The alarming antimicrobial resistance in ESKAPEE pathogens: Can essential oils come to the rescue? Fitoterapia 2020, 40, 104433. [Google Scholar] [CrossRef]
- Cassini, A.; Hogberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Touat, M.; Opatowski, M.; Brun-Buisson, C.; Cosker, K.; Guillemot, D.; Salomon, J.; Tuppin, P.; de Lagasnerie, G.; Watier, L. A payer perspective of the hospital inpatient additional care costs of antimicrobial resistance in France: A matched case–control study. Appl. Health Econ. Health Policy 2019, 17, 381–389. [Google Scholar] [CrossRef] [Green Version]
- European Comission. EU Action on Antimicrobial Resistance. ECDC: Surveillance of Antimicrobial Resistance in Europe 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018 (accessed on 9 February 2020).
- Shanmugaraj, B.; Malla, A.; Phoolcharoen, W. Emergence of Novel Coronavirus 2019-nCoV: Need for Rapid Vaccine and Biologics Development. Pathogens 2020, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections—United States. Morb. Mortal. Wkly. Rep. 2019, 68, 214. [Google Scholar] [CrossRef] [Green Version]
- Mir, M.; Ahmed, N.; Permana, A.D.; Rodgers, A.M.; Donnelly, R.F.; Rehman, A. Enhancement in Site-Specific Delivery of Carvacrol against Methicillin Resistant Staphylococcus aureus Induced Skin Infections Using Enzyme Responsive Nanoparticles: A Proof of Concept Study. Pharmaceutics 2019, 11, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konai, M.M.; Bhattacharjee, B.; Ghosh, S.; Haldar, J. Recent Progress in Polymer Research to Tackle Infections and Antimicrobial Resistance. Biomacromolecules 2018, 19, 1888–1917. [Google Scholar] [CrossRef] [PubMed]
- Francolini, I.; Piozzi, A. Polymeric Systems as Antimicrobial or Antifouling Agents. Int. J. Mol. Sci. 2019, 20, 4866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Villegas, N.A.; Ravetti, S.; Bermu’dez, J.M.; Cid, A.G.; Allemandi, D.A.; Palma, S.D. Metallic nanoparticles as a strategy for the treatment of infectious diseases. In Materials for Biomedical Engineering: Bioactive Materials for Antimicrobial, Anticancer, and Gene Therapy, 1st ed.; Holban, A.M., Grumezescu, A.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Chapter 14; pp. 383–407. [Google Scholar]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [Green Version]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Kalan, L.; Grice, E.A. Fungi in the Wound Microbiome. Adv. Wound Care 2018, 7, 247–255. [Google Scholar] [CrossRef]
- Maccelli, A.; Vitanza, L.; Imbriano, A.; Fraschetti, C.; Filippi, A.; Goldoni, P.; Maurizi, L.; Ammendolia, M.G.; Crestoni, M.E.; Fornarini, S.; et al. Satureja montana L. Essential Oils: Chemical Profiles/Phytochemical Screening, Antimicrobial Activity and O/WNanoEmulsion Formulations. Pharmaceutics 2020, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef]
- Baygar, T.; Sarac, N.; Ugur, A.; Karaca, I.R. Antimicrobial characteristics and biocompatibility of the surgical sutures coated with biosynthesized silver nanoparticles. Bioorganic Chem. 2019, 86, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.L.; Paladini, F.; Romano, A.; Verri, T.; Quattrini, A.; Sannino, A.; Pollini, M. Efficacy of silver coated surgical sutures on bacterial contamination, cellular response and wound healing. Mat. Sci. Eng. 2016, 69, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; George, A.; Gopi, S.; Kalarikkal, N.; Thomas, S. Polymer sutures for simultaneous wound healing and drug delivery—A review. Int. J. Pharmac. 2017, 524, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, P.; Tandon, M. Antimicrobial properties of iodine based products. J. Sci. Ind. Res. 2010, 69, 376–383. [Google Scholar]
- Kaiho, T. Iodine Chemistry and Applications, 1st ed.; Kaiho, T., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 15–410, ISBN-13 978-1-118-46629-2. [Google Scholar]
- He, C.; Parrish, D.A.; Shreeve, J.M. Alkyl ammonium cation stabilized biocidal polyiodides with adaptable high density and low pressure. Chem. Eur. J. 2014, 20, 6699–6706. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, C.; Zhou, W.; Hooper, J.P.; Imler, G.H.; Parrish, D.A.; Shreeve, J.M. Control of Biohazards: A High Performance Energetic Polycyclized Iodine-Containing Biocide. Inorg. Chem. 2018, 57, 8673–8680. [Google Scholar] [CrossRef]
- Manchanda, G.; Sodhi, R.K.; Jain, U.K.; Chandra, R.; Madan, J. Iodinated curcumin bearing dermal cream augmented drug delivery, antimicrobial and antioxidant activities. J. Microencapsul. 2018, 35, 49–61. [Google Scholar] [CrossRef]
- Viswanathan, K.; Babu, D.B.; Jayakumar, G.; Raj, G.D. Anti-microbial and skin wound dressing application of molecular iodine nanoparticles. Mater. Res. Express 2017, 4, 104003. [Google Scholar] [CrossRef]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.-K.; Wa, C.T.C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef]
- Svensson, P.H.; Kloo, L. Synthesis, structure, and bonding in polyiodide and metal iodide−iodine systems. Chem. Rev. 2003, 103, 1649–1684. [Google Scholar] [CrossRef]
- Blake, A.J.; Li, W.S.; Lippolis, V.; Schröder, M.; Devillanova, F.A.; Gould, R.O.; Parsons, S.; Radek, C. Template self-assembly of polyiodide networks. Chem. Soc. Rev. 1998, 27, 195–206. [Google Scholar] [CrossRef]
- Edis, Z.; Bloukh, S.H. Preparation and structural and spectroscopic characterization of a pentaiodide [Rb(12-crown-4)2]I5. Z. Nat. 2013, 68, 1340–1346. [Google Scholar]
- Van Mengen, M.; Reiss, G.J. I62− Anion composed of two asymmetric triiodide moieties: A competition between halogen and hydrogen bond. Inorganics 2013, 1, 3–13. [Google Scholar] [CrossRef]
- Reiss, G.J. Halogen and hydrogen bonding in the layered crystal structure of 2-iodoaniliniumtriiodide, C6H7I4N. Z. Kristallogr. NCS 2019, 234, 899–902. [Google Scholar]
- Wen, X.; Zhang, X.; Szewczyk, G.; El-Hussein, A.; Huang, Y.-Y.; Sarna, T.; Hamblin, M.R. Potassium iodide potentiates antimicrobial photodynamic inactivation mediated by rose bengal in in vitro and in vivo studies. Antimicrob. Agents Chemother. 2017, 61, e00467-17. [Google Scholar] [CrossRef] [Green Version]
- Vieira, C.; Gomes, A.T.P.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An Insight into the Potentiation Effect of Potassium Iodide on aPDT Efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartashevich, E.V.; Grigoreva, E.A.; Yushina, I.D.; Bulatova, L.M.; Tsirelson, V.G. Modern level for the prediction of properties of iodine-containing organic compounds: Iodine forming halogen bonds. Russ. Chem. Bull. Int. Ed. 2017, 66, 1–12. [Google Scholar] [CrossRef]
- Edis, Z.; Bloukh, S.H. Preparation and structural and spectroscopic characterization of triiodides [M(12-crown-4)2]I3 with M = Na and Rb. Z. Nat. 2014, 69, 995–1002. [Google Scholar]
- Edis, Z.; Haj Bloukh, S.; Abu Sara, H.; Bhakhoa, H.; Rhyman, L.; Ramasami, P. “Smart” triiodide compounds: Does halogen bonding influence antimicrobial activities? Pathogens 2019, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Haj Bloukh, S.; Edis, Z. Halogen bonding in Crystal structure of bis(1,4,7,10-tetraoxacyclododecane-κ4O,O′,O′′,O′′′)cesium triiodide, C16H32CsI3O8. Z. Kristallogr. NCS 2020, in press. [Google Scholar] [CrossRef] [Green Version]
- Haj Bloukh, S.; Edis, Z. Structure and Antimicrobial properties of bis(1,4,7,10-tetraoxacyclododecane-κ4O,O′,O′′,O′′′)cesium pentaiodide, C16H32CsI5O8. Z. Kristallogr. NCS 2020, in press. [Google Scholar] [CrossRef] [Green Version]
- Arora, S.; Yadav, V.; Kumar, P.; Gupta, R.; Kumar, D. Polymer based antimicrobial coatings as potential biomaterial: A review. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 279–290. [Google Scholar]
- Danilovas, P.P.; Rutkaite, R.; Zemaitaitis, A. Thermal degradation and stability of cationic starches and their complexes with iodine. Carbohydr. Polym. 2014, 112, 721–728. [Google Scholar] [CrossRef]
- Some, S.; Sohn, J.S.; Kim, J.; Lee, S.-H.; Lee, S.C.; Lee, J.; Shackery, I.; Kim, S.K.; Kim, S.H.; Choi, N.; et al. Graphene-Iodine Nanocomposites: Highly Potent Bacterial Inhibitors that are Bio-compatible with Human Cells. Sci. Rep. 2015, 6, 20015. [Google Scholar] [CrossRef]
- Gao, T.; Fan, H.; Wang, X.; Gao, Y.; Liu, W.; Chen, W.; Dong, A.; Wang, Y.J. Povidone-Iodine-Based Polymeric Nanoparticles for Antibacterial applications. ACS Appl. Mater. Interfaces 2017, 9, 25738–25746. [Google Scholar] [CrossRef]
- Schmitz, G.; Rosenblatt, L.; Salerno, N.; Odette, J.; Ren, R.; Emanuel, T.; Michalek, J.; Liu, Q.; Du, L.; Jahangir, K.; et al. Treatment data using a topical povidone-iodine antiseptic in patients with superficial skin abscesses. Data Brief 2019, 23, 103715. [Google Scholar] [CrossRef]
- Goodwin, M.J.; Steed, B.W.; Yufit, D.S.; Musa, O.M.; Berry, D.J.; Steed, J.W. Halogen and Hydrogen Bonding in Povidone-Iodine and Related Co-Phases. Cryst. Growth Des. 2017, 17, 5552–5558. [Google Scholar] [CrossRef] [Green Version]
- Mamatha, G.; Rajulu, A.V.; Madhukar, K. Development and analysis of cellulose nanocomposite films with in situ generated silver nanoparticles using tamarind nut powder as a reducing agent. Int. J. Polymer. A Charact. 2019, 24, 219–226. [Google Scholar] [CrossRef]
- Edis, Z.; Haj Bloukh, S.; Ashames, A.; Ibrahim, M. Copper-Based Nanoparticles, their chemistry and Antibacterial properties: A Review. In Chemistry for a Clean and Healthy Planet, 1st ed.; Ramasami, P., Gupta Bhowon, M., Jhaumeer Laulloo, S., Li Kam Wah, H., Eds.; Springer Nature AG: Cham, Switzerland, 2019; pp. 401–428, ISBN-13 978-3-030-20282-8. [Google Scholar]
- Reda, M.; Ashames, A.; Edis, Z.; Bloukh, S.; Bhandare, R.; Abu Sara, H. Green Synthesis of Potent Antimicrobial Silver Nanoparticles Using Different Plant Extracts and Their Mixtures. Processes 2019, 7, 510. [Google Scholar] [CrossRef] [Green Version]
- Su-Eon Jin, S.-E.; Jin, H.-E. Synthesis, Characterization, and Three-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications. Pharmaceutics 2019, 11, 575. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Buabeid, M.A.; Arafa, E.A.; Hussain, I.; Lid, L.; Murtaza, G. The wound healing and antibacterial potential of triple-component nanocomposite (chitosan-silver-sericin) films loaded with moxifloxacin. Int. J. Pharm. 2019, 564, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Ashames, A.A.; Buabeid, M.A.; Murtaza, G. Synthesis, in vitro characterization and antibacterial efficacy of moxifloxacin-loaded chitosan-pullulan-silver-nanocomposite films. J. Drug Delivery Sci. Tech. 2020, 55, 101366–101383. [Google Scholar] [CrossRef]
- Kanwar, R.; Rathee, J.; Salunke, D.B.; Mehta, S.K. Green Nanotechnology-Driven Drug Delivery Assemblies. ACS Omega 2019, 4, 8804–8815. [Google Scholar] [CrossRef] [Green Version]
- Riau, A.K.; Aung, T.T.; Setiawan, M.; Yang, L.; Yam, G.H.F.; Beuerman, R.W.; Venkatraman, S.S.; Mehta, J.S. Surface Immobilization of Nano-Silver on Polymeric Medical Devices to Prevent Bacterial Biofilm Formation. Pathogens 2019, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Sang Hun Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Burlacu, E.; Tanase, C.; Coman, N.-A.; Berta, L. A Review of Bark-Extract-Mediated Green Synthesis of Metallic Nanoparticles and Their Applications. Molecules 2019, 24, 4354. [Google Scholar] [CrossRef] [Green Version]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Premkumar, J.; Sudhakar, T.; Dhakal, A.; Shrestha, J.B.; Krishnakumar, S.; Balashanmugam, P. Synthesis of silver nanoparticles (AgNPs) from cinnamon against bacterial Pathogens. Biocat. Agric. Biotech. 2018, 15, 311–316. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.A.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Compl. Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef] [Green Version]
- Soni, N.; Prakash, S. Green Nanoparticles for Mosquito Control. Sci. World J. 2014, 2014, 496362. [Google Scholar] [CrossRef] [Green Version]
- Malheiro, J.F.; Maillard, J.-Y.; Borges, F.; Simões, M. Biocide Potentiation Using Cinnamic Phytochemicals and Derivatives. Molecules 2019, 24, 3918. [Google Scholar] [CrossRef] [Green Version]
- Malheiro, J.F.; Gomes, I.; Borges, A.; Bastos, M.M.S.M.; Maillard, J.Y.; Borges, F.; Simões, M. Phytochemical profiling as a solution to palliate disinfectant limitations. Biofouling 2016, 32, 1007–1016. [Google Scholar] [CrossRef]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef]
- Letsididi, K.S.; Lou, Z.; Letsididi, R.; Mohammed, K.; Maguy, B.L. Antimicrobial and antibiofilm effects of trans-cinnamic acid nanoemulsion and its potential application on lettuce. LWT Food. Sci. Technol. 2018, 94, 25–32. [Google Scholar] [CrossRef]
- Anwar, A.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Gold nanoparticle-conjugated cinnamic acid exhibits antiacanthamoebic and antibacterial properties. Antimicrob. Agents Chemother. 2018, 62, e00630-18. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, K.; Anwar, A.; Khan, N.A.; Shah, M.R.; Siddiqui, R. Trans-Cinnamic Acid Conjugated Gold Nanoparticles as Potent Therapeutics against Brain-Eating Amoeba Naegleria fowleri. ACS Chem. Neurosci. 2019, 10, 2692–2696. [Google Scholar] [CrossRef]
- Zhao, Q.; Luan, X.; Zheng, M.; Tian, X.-H.; Zhao, J.; Zhang, W.-D.; Ma, B.-L. Synergistic Mechanisms of Constituents in Herbal Extracts during Intestinal Absorption: Focus on Natural Occurring Nanoparticles. Pharmaceutics 2020, 12, 128. [Google Scholar] [CrossRef] [Green Version]
- Küünal, S.; Rauwel, P.; Rauwel, E. Plant extract mediated synthesis of nanoparticles. In Emerging Applications of Nanoparticles and Architecture Nanostructures: Current Prospects and Future Trends, Micro and Nano Technologies, 1st ed.; Makhlouf, A.S.H., Barhoum, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Chapter 14; pp. 411–446, eBook ISBN 9780128135167, Paperback ISBN 9780323512541. [Google Scholar]
- Logaranjan, K.; Raiza, A.J.; Gopinath, S.C.B.; Chen, Y.; Pandian, K. Shape- and Size-Controlled Synthesis of Silver Nanoparticles Using Aloe vera Plant Extract and Their Antimicrobial Activity. Nanoscale Res. Lett. 2016, 11, 520–529. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.W.; Perry, D.M.; Kirby, W.M.M. Single-disk antibiotic-sensitivity testing of staphylococci: An analysis of technique and results. AMA Arch. Intern. Med. 1959, 104, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Testing, 28th ed.; M100S; CLSI: Wayne, PA, USA, 2018; Volume 38. [Google Scholar]
- Khan, A.U.; Khan, M.; Khan, M.M. Antifungal and Antibacterial Assay by Silver Nanoparticles Synthesized from Aqueous Leaf Extract of Trigonella foenum-graecum. BioNanoScience 2019, 9, 597–602. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Hussein, S.A.; Ahmed, H.M. Effect of PVA Blending on Structural and Ion Transport Properties of CS:AgNt-Based Polymer Electrolyte Membrane. Polymers 2017, 9, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brza, M.A.; Aziz, S.B.; Anuar, H.; Al Hazza, M.H.F. From Green Remediation to Polymer Hybrid Fabrication with Improved Optical Band Gaps. Int. J. Mol. Sci. 2019, 20, 3910. [Google Scholar] [CrossRef] [Green Version]
- Al Aboody, M.S. Silver/silver chloride (Ag/AgCl) nanoparticles synthesized from Azadirachta indica lalex and its antibiofilm activity against fluconazole resistant Candida tropicalis. Art. Cells Nanomed. Biotech. 2019, 47, 2107–2113. [Google Scholar] [CrossRef] [Green Version]
- Dhafer, C.E.B.; Dhahri, M.; Mezni, A.; Smiri, L.S.S. Surface - enhanced Raman scattering study of PP/Ag nanocomposite developed to prevent postsurgery infection. J. RAMAN Spectrosc. 2018, 49, 1445–1451. [Google Scholar] [CrossRef]
- Kora, A.J.; Arunachalam, J. Green Fabrication of Silver Nanoparticles by Gum Tragacanth (Astragalus gummifer): A Dual Functional Reductant and Stabilizer. J. Nanomat. 2012, 2012, 869765. [Google Scholar] [CrossRef] [Green Version]
- Malheiro, J.; Maillard, J.; Borges, F.; Simões, M. Evaluation of cinnamaldehyde and cinnamic acid derivatives in microbial growth control. Int. Biodeterior. Biodegrad. 2018, 141, 71–78. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark and clove bud extracts) for antimicrobial delivery applications. LWT Food. Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- De Simone, S.; Gallo, A.L.; Paladini, F.; Sannino, A.; Pollini, M. Development of silver nano-coatings on silk sutures as a novel approach against surgical infections. J. Mater. Sci. Mater. Med. 2014, 25, 2205–2214. [Google Scholar] [CrossRef]
- Blaker, J.J.; Nazhat, S.N.; Boccaccini, A.R. Development and characterisation of silver-doped bioactive glass-coated sutures for tissue engineering and wound healing applications. Biomaterials 2004, 25, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Sunitha, S.; Adinarayana, K.; Sravanthi, R.P.; Sonia, G.; Nagarjun, R.; Pankaj, T.; Veerabhadra, S.C.; Sujatha, D. Fabrication of Surgical Sutures Coated with Curcumin Loaded Gold Nanoparticles. Pharm. Anal. Acta 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Ashour, A.A.; Raafat, D.; El-Goweili, H.M.; El-Kamel, A.H. Green synthesis of silver nanoparticles using cranberry powder aqueous extract: Characterization and antimicrobial properties. Int. J. Nanomed. 2015, 10, 7207–7221. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Sun, Y.; Che, G.; Li, Z. Self-assembled silver nanoparticle films at an air—Liquid interface and their applications in SERS and electrochemistry. Appl. Surf. Sci. 2011, 257, 7150–7155. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Won, S.W.; Cho, C.W.; Kim, S.; Yun, Y.S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloid. Surf. B Biointerface 2009, 73, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Balashanmugam, P.; Kalaichelvan, P.T. Biosynthesis characterization of silver nanoparticles using Cassia roxburghii DC. aqueous extract and coated on cotton cloth for effective antibacterial activity. Int. J. Nanomed. 2015, 1, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, H.M.; Mosleh, A.M.; Elghany, A.A.; Shams-Eldin, E.; Abu Serea, E.S.; Ali, S.A.; Shalan, A.E. Coated silver nanoparticles: Synthesis, cytotoxicity, and optical properties. RSC Adv. 2019, 9, 20118–20136. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Yang, D.C. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif. Cells. Nanomed. Biotechnol. 2016, 44, 1150–1157. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Devi, P.; Kumar, A. Structural analysis of PVP capped silver nanoparticles synthesized at room temperature for optical, electrical and gas sensing properties. J. Mater. Sci. Mater. Elect. 2017, 28, 5014–5020. [Google Scholar] [CrossRef]
- Kirillov, S.A. Novel Approaches in Spectroscopy of Interparticle Interactions, Vibrational Line Profiles and Anomalous Non-Coincidence Effects. In Novel Approaches to the Structure and Dynamics of Liquids: Experiments, Theories and Simulations, 1st ed.; NATO Science Series (Series II: Mathematics, Physics and Chemistry); Samios, J., Durov, V.A., Eds.; Springer: Dortrecht, The Netherlands, 2004; Volume 133, pp. 193–227, ISBN-13 978-1-4020-1847-3. [Google Scholar]
- Aguilar-Hernández, I.; Afseth, N.K.; López-Luke, T.; Contreras-Torres, F.F.; Wold, J.P.; Ornelas-Soto, N. Surface enhanced Raman spectroscopy of phenolic antioxidants: A systematic evaluation of ferulic acid, p-coumaric acid, caffeic acid and sinapic acid. Vibr. Spectr. 2017, 89, 113–122. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kamal, S.K.; Kumar, T.J.; Sreedhar, B.; Singh, A.K.; Srivastava, S.K. Synthesis of Silver Nanoparticles using Facile Wet Chemical Route. Def. Sci. J. 2009, 59, 447–455. [Google Scholar] [CrossRef]
- Kumar, V.A.; Uchida, T.; Mizuki, T.; Nakajima, Y.; Katsube, Y.; Hanajiri, T.; Maekawa, T. Synthesis of nanoparticles composed of silver and silver chloride for a plasmonic photocatalyst using an extract from a weed Solidago altissima (goldenrod). Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 015002. [Google Scholar] [CrossRef]
- Hamed, S.M.; Mostafa, A.M.A.; Abdel-Raouf, N.; Ibraheem, I.B.M. Biosynthesis of silver and silver chloride nanoparticles by Parachlorella kessleri SAG 211-11 and evaluation of its nematicidal potential against the root-knot nematode; Meloidogyne incognita. Aust. J. Basic Appl. Sci. 2016, 10, 354–364. [Google Scholar]
- Ellison, J.; Wykoff, G.; Paul, A.; Mohseni, R.; Vasiliev, A. Efficient dispersion of coated silver nanoparticles in the polymer matrix. Colloid. Surf. A. Physicochem. Eng. Asp. 2014, 447, 67–70. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.Q.; Shaifur Rahman, M.D.; Rahaman, M.S.; Shajahan, M.; Dafader, N.C. Improvement of Swelling Behaviour of Poly (Vinyl Pyrrolidone) and Acrylic Acid Blend Hydrogel Prepared By the Application of Gamma Radiation. Org. Chem. Curr. Res. 2015, 4, 2. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Abdelghany, A.M.; Badr, S.I.; Morsi, M.A. Structural, optical, morphological and thermal properties of PEO/PVP blend containing different concentrations of biosynthesized Au nanoparticles. J. Mater. Res. Technol. 2018, 7, 419–431. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Xing, B.; Mukherjee, A.; Musante, C.; White, J.C.; He, L. Analysis of Silver Nanoparticles in Antimicrobial Products Using Surface-Enhanced Raman Spectroscopy (SERS). Environ. Sci. Technol. 2015, 49, 4317–4324. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.R.P.S.; Rafael, O.; Correa, R.O.; Stroppa, P.H.F.; Flavia, C.; Marques, F.C.; Gustavo, F.S.; Andrade, G.F.S.; Correa, C.C.; Brandao, M.A.F.; et al. Biosynthesis of silver nanoparticles using Caesalpinia ferrea (Tul.) Martius extract: Physicochemical characterization, antifungal activity and cytotoxicity. PeerJ 2018, 6, e4361. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Walkenfort, B.; Yoon, J.H.; Schlucker, S.; Xie, W. Gold and silver nanoparticle monomers are non-SERS-active: A negative experimental study with silica-encapsulated Raman-reporter-coated metal colloids. Phys. Chem. Chem. Phys. 2015, 17, 21120–21126. [Google Scholar] [CrossRef] [Green Version]



















| Capping Agent Used | Zeta-Potential (mV) | Particle Size Mean (nm) | Poly-Dispersity Index (PDI) |
|---|---|---|---|
| TCA-AgNP | −17.9 | 61.3 ± 11.2 | 0.390 |
| TCA-AgNP-PI | −33.3 | 57.2 ± 10.4 | 0.464 |
| Cinn-AgNP | −32.9 | 88.1 ± 20.6 | 0.376 |
| Cinn-AgNP-PI | −33.1 | 51.5 ± 7.8 | 0.486 |
| Strain | Anti-Biotic | A | 1W | 2W | 3W | 4W | 1D | 2D | 3D | 4D | 1D* | 2D* | 3D* | 4D* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. pneumoniae ATCC 49619 | G | 21 | 16 | 17 | 0 | 0 | 14 | 13 | 0 | 13 | 10 | 10 | 0 | 0 |
| S. aureus ATCC 25923 | G | 27 | 17 | 21 | 14 | 20 | 16 | 15 | 10 | 13 | 14 | 10 | 9 | 12 |
| E. faecalis ATCC 29212 | CTX | 25 | 13 | 15 | 10 | 11 | 12 | 12 | 0 | 12 | 9 | 0 | 0 | 0 |
| P. aeruginosa WDCM 00026 | CTX | 20 | 20 | 27 | 15 | 15 | 20 | 20 | 10 | 14 | 13 | 13 | 0 | 11 |
| E. coli WDCM 00013 | G | 23 | 17 | 17 | 14 | 14 | 17 | 18 | 11 | 12 | 13 | 13 | 10 | 10 |
| C. albicans WDCM 00054 | NY | 16 | 18 | 13 | 0 | 15 | 13 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| Strain | Anti-Biotic | A | 1S | 2S | 3S | 4S |
|---|---|---|---|---|---|---|
| S. pneumoniae ATCC 49619 | G | 21 | 0 | 0 | 4 | 0 |
| S. aureus ATCC 25923 | G | 27 | 1 | 5 | 1 | 1 |
| E. faecalis ATCC 29212 | CT | 25 | 0 | 0 | 0 | 0 |
| S. pyogenes ATCC 19615 | C | 25 | 2 | 2.5 | 0.5 | 0 |
| B. subtilis | S | 20 | 4 | 5 | 0 | 0 |
| P. aeruginosa WDCM 00026 | CTX | 20 | 5 | 5 | 3 | 1 |
| E. coli WDCM 00013 | G | 23 | 5 | 5 | 0 | 4 |
| K. pneumoniae WDCM 00097 | CTX | 35 | 4 | 5 | 5 | 1 |
| P. mirabilis ATCC 29906 | G | 35 | 1 | 1 | 0 | 1 |
| C. albicans WDCM 00054 | NY | 16 | 0 | 0 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edis, Z.; Haj Bloukh, S.; Ibrahim, M.R.; Abu Sara, H. “Smart” Antimicrobial Nanocomplexes with Potential to Decrease Surgical Site Infections (SSI). Pharmaceutics 2020, 12, 361. https://doi.org/10.3390/pharmaceutics12040361
Edis Z, Haj Bloukh S, Ibrahim MR, Abu Sara H. “Smart” Antimicrobial Nanocomplexes with Potential to Decrease Surgical Site Infections (SSI). Pharmaceutics. 2020; 12(4):361. https://doi.org/10.3390/pharmaceutics12040361
Chicago/Turabian StyleEdis, Zehra, Samir Haj Bloukh, May Reda Ibrahim, and Hamed Abu Sara. 2020. "“Smart” Antimicrobial Nanocomplexes with Potential to Decrease Surgical Site Infections (SSI)" Pharmaceutics 12, no. 4: 361. https://doi.org/10.3390/pharmaceutics12040361
APA StyleEdis, Z., Haj Bloukh, S., Ibrahim, M. R., & Abu Sara, H. (2020). “Smart” Antimicrobial Nanocomplexes with Potential to Decrease Surgical Site Infections (SSI). Pharmaceutics, 12(4), 361. https://doi.org/10.3390/pharmaceutics12040361