Salvianolic Acid B in Microemulsion Formulation Provided Sufficient Hydration for Dry Skin and Ameliorated the Severity of Imiquimod-induced Psoriasis-like Dermatitis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microemulsion Preparation
2.2.1. Formulation Selection
2.2.2. Stability Study
2.3. Microemulsion Characterization
2.3.1. Measurement of Droplet Size and Zeta Potential
2.3.2. Electronic Conductivity
2.3.3. Viscosity
2.4. Animals
Skin Irritation and Study Design
2.5. Preparation of Mice Skin for In-vitro Skin Permeation and Deposition Studies
2.6. In-vitro Skin Permeation Study
2.7. Permeation Data Analysis
2.8. Skin Deposition Study
2.9. HPLC System
2.10. Barrier Function Study
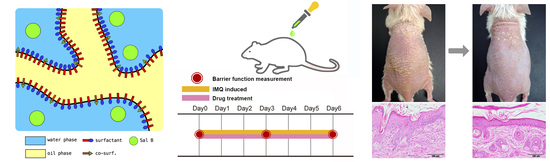
2.10.1. Imiquimod-induced Psoriasis-like Skin Animal Model
2.10.2. Assessment of Barrier Function
2.11. Collection of Skin Specimens
2.12. Inflammatory Cytokine Protein Determination
2.13. Proliferation Cell Nuclear Antigen (PCNA) Protein Determination
2.14. Histological Staining
2.15. Statistical Analysis
3. Results
3.1. Formulation Composition and Characterization of Selected Formulation
3.2. Skin Penetration Parameters and Deposition Amounts of Selected Formulations
3.3. Formulation A Was Selected as the Topical Delivery Carrier for Sal. B
3.4. Sal. B/Formulation A and 0.25% DXM Restored Barrier Function
3.5. Sal. B/Formulation A and DXM Improve Psoriasis-like Dermatitis
3.6. Sal. B/Formulation A and DXM Inhibit IL-23, IL-17A, IL-17F, and IL-22 Protein Expression in Psoriasis-like Skin
3.7. Sal. B/Formulation A and DXM Inhibit Hyperproliferation of Keratinocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Azfar, R.S.; Gelfand, J.M. Psoriasis and metabolic disease: Epidemiology and pathophysiology. Curr. Opin. Rheumatol. 2008, 20, 416–422. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Di Cesare, A.; Di Meglio, P.; Nestle, F.O. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J. Investig. Dermatol. 2009, 129, 1339–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toussirot, E. The IL23/Th17 pathway as a therapeutic target in chronic inflammatory diseases. Inflamm. Allergy Drug Targets 2012, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Qian, S.S.; Zhang, Y.J.; Wang, R.Q. Salvia miltiorrhiza: A source for anti-Alzheimer’s disease drugs. Pharm. Biol. 2016, 54, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habtemariam, S. Molecular Pharmacology of Rosmarinic and Salvianolic Acids: Potential Seeds for Alzheimer’s and Vascular Dementia Drugs. Int. J. Mol. Sci. 2018, 19, E1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, M.; Huang, F.; Deng, C.; Wang, Y.; Kai, G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 2019, 59, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Guo, X.W.; Zheng, H.Z.; Li, D.P.; Jia, G.B.; Wang, J. Current progress of research on pharmacologic actions of salvianolic acid B. Chin. J. Integr. Med. 2012, 18, 316–320. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, Z.; Chow, M.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef]
- Lin, J.; Lin, R.; Li, S.; Wu, H.; Ding, J.; Xiang, G.; Li, S.; Wang, Y.; Lin, D.; Gao, W.; et al. Salvianolic Acid B Promotes the Survival of Random-Pattern Skin Flaps in Rats by Inducing Autophagy. Front. Pharmacol. 2018, 9, 1178. [Google Scholar] [CrossRef] [Green Version]
- Lay, I.S.; Hsieh, C.C.; Chiu, J.H.; Shiao, M.S.; Lui, W.Y.; Wu, C.W. Salvianolic acid B enhances in vitro angiogenesis and improves skin flap survival in Sprague-Dawley rats. J. Surg. Res. 2003, 115, 279–285. [Google Scholar] [CrossRef]
- Shoba, E.; Lakra, R.; Syamala Kiran, M.; Korrapati, P.S. Fabrication of core-shell nanofibers for controlled delivery of bromelain and salvianolic acid B for skin regeneration in wound therapeutics. Biomed. Mater. 2017, 12, 035005. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Park, S.Y.; Hwang, E.; Zhang, M.; Jin, F.; Zhang, B.; Yi, T.H. Salvianolic Acid B Protects Normal Human Dermal Fibroblasts Against Ultraviolet B Irradiation-Induced Photoaging Through Mitogen-Activated Protein Kinase and Activator Protein-1 Pathways. Photochem. Photobiol. 2015, 91, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Nastiti, C.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, E37. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Watkinson, R.M.; Herkenne, C.; Guy, R.H.; Hadgraft, J.; Oliveira, G.; Lane, M.E. Influence of ethanol on the solubility, ionization and permeation characteristics of ibuprofen in silicone and human skin. Skin Pharmacol. Physiol. 2009, 22, 15–21. [Google Scholar] [CrossRef]
- Cartner, T.; Brand, N.; Tian, K.; Saud, A.; Carr, T.; Stapleton, P.; Lane, M.E.; Rawlings, A.V. Effect of different alcohols on stratum corneum kallikrein 5 and phospholipase A2 together with epidermal keratinocytes and skin irritation. Int. J. Cosmet. Sci. 2017, 39, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Jacobi, U.; Bartoll, J.; Sterry, W.; Lademann, J. Orally administered ethanol: Transepidermal pathways and effects on the human skin barrier. Arch. Dermatol. Res. 2005, 296, 332–338. [Google Scholar] [CrossRef]
- Loffler, H.; Kampf, G.; Schmermund, D.; Maibach, H.I. How irritant is alcohol? Br. J. Dermatol. 2007, 157, 74–81. [Google Scholar] [CrossRef]
- Celleno, L. Topical urea in skincare: A review. Dermatol. Ther. 2018, 31, e12690. [Google Scholar] [CrossRef] [PubMed]
- Loden, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Madison, K.C. Barrier function of the skin: “la raison d’etre” of the epidermis. J. Investig. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bikowski, J. The use of therapeutic moisturizers in various dermatologic disorders. Cutis 2001, 68, 3–11. [Google Scholar] [PubMed]
- Ryu, K.A.; Park, P.J.; Kim, S.B.; Bin, B.H.; Jang, D.J.; Kim, S.T. Topical Delivery of Coenzyme Q10-Loaded Microemulsion for Skin Regeneration. Pharmaceutics 2020, 12, 332. [Google Scholar] [CrossRef] [Green Version]
- Horvath, S.; Komlodi, R.; Perkecz, A.; Pinter, E.; Gyulai, R.; Kemeny, A. Methodological refinement of Aldara-induced psoriasiform dermatitis model in mice. Sci. Rep. 2019, 9, 3685. [Google Scholar] [CrossRef] [Green Version]
- Chong, H.T.; Yang, G.N.; Sidhu, S.; Ibbetson, J.; Kopecki, Z.; Cowin, A.J. Reducing Flightless I expression decreases severity of psoriasis in an imiquimod-induced murine model of psoriasiform dermatitis. Br. J. Dermatol. 2017, 176, 705–712. [Google Scholar] [CrossRef]
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Wang, P.C.; Huang, Y.L.; Hou, S.S.; Chou, C.H.; Tsai, J.C. Lauroyl/palmitoyl glycol chitosan gels enhance skin delivery of magnesium ascorbyl phosphate. J. Cosmet. Sci. 2013, 64, 273–286. [Google Scholar]
- Sekhon, S.; Jeon, C.; Nakamura, M.; Afifi, L.; Yan, D.; Wu, J.J.; Liao, W.; Bhutani, T. Review of the mechanism of action of coal tar in psoriasis. J. Dermatolog. Treat. 2018, 29, 230–232. [Google Scholar] [CrossRef]
- Blum, P.; Sagner, A.; Tiehm, A.; Martus, P.; Wendel, T.; Grathwohl, P. Importance of heterocylic aromatic compounds in monitored natural attenuation for coal tar contaminated aquifers: A review. J. Contam. Hydrol. 2011, 126, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.R.; Zong, A.B.; Taguchi, A.T.; Nocera, D.G.; Stubbe, J.; Tommos, C. Formal Reduction Potentials of Difluorotyrosine and Trifluorotyrosine Protein Residues: Defining the Thermodynamics of Multistep Radical Transfer. J. Am. Chem. Soc. 2017, 139, 2994–3004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamsuzzaman, K.H. Bioactive Benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef]
- Szpiech, Z.A.; Mak, A.C.Y.; White, M.J.; Hu, D.; Eng, C.; Burchard, E.G.; Hernandez, R.D. Ancestry-Dependent Enrichment of Deleterious Homozygotes in Runs of Homozygosity. Am. J. Hum. Genet. 2019, 105, 747–762. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.M.; Ma, M.; Li, H.; Qi, Q.; Liu, Y.T.; Yan, Y.X.; Shen, Y.F.; Yang, X.Q.; Zhu, F.H.; He, S.J.; et al. Topical administration of reversible SAHH inhibitor ameliorates imiquimod-induced psoriasis-like skin lesions in mice via suppression of TNF-alpha/IFN-gamma-induced inflammatory response in keratinocytes and T cell-derived IL-17. Pharmacol. Res. 2018, 129, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Du, Z.Y.; Li, P.H.; Yan, L.; Zhou, W.; Tang, Y.D.; Liu, G.R.; Fang, Y.X.; Zhang, K.; Dong, C.Z.; et al. Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol. 2018, 64, 319–325. [Google Scholar] [CrossRef]
- OuYang, Q.; Pan, Y.; Luo, H.; Xuan, C.; Liu, J.; Liu, J. MAD ointment ameliorates Imiquimod-induced psoriasiform dermatitis by inhibiting the IL-23/IL-17 axis in mice. Int. Immunopharmacol. 2016, 39, 369–376. [Google Scholar] [CrossRef]
- Francesco, B.; Paolo, G.; Giampiero, G. A dermatologist perspective in the pharmacological treatment of patients with psoriasis and psoriatic arthritis. Expert Rev. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Rendon, A.; Schakel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [Green Version]
- Campanati, A.; Diotallevi, F.; Martina, E.; Paolinelli, M.; Radi, G.; Offidani, A. Safety update of etanercept treatment for moderate to severe plaque psoriasis. Expert Opin. Drug Saf. 2020, 19, 439–448. [Google Scholar] [CrossRef]
- Elias, P.M.; Wakefield, J.S.; Man, M.Q. Moisturizers versus Current and Next-Generation Barrier Repair Therapy for the Management of Atopic Dermatitis. Skin Pharmacol. Physiol. 2019, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Man, M.Q.; Elias, P.M. Could Inflammaging and Its Sequelae Be Prevented or Mitigated? Clin. Interv. Aging 2019, 14, 2301–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fluhr, J.W.; Cavallotti, C.; Berardesca, E. Emollients, moisturizers, and keratolytic agents in psoriasis. Clin. Dermatol. 2008, 26, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E. The role of emollients in the management of diseases with chronic dry skin. Skin Pharmacol. Physiol. 2008, 21, 75–80. [Google Scholar] [CrossRef]
- Luger, T.; Seite, S.; Humbert, P.; Krutmann, J.; Triller, R.; Dreno, B. Recommendations for adjunctive basic skin care in patients with psoriasis. Eur. J. Dermatol. 2014, 24, 194–200. [Google Scholar] [CrossRef]
- Man, M.Q.; Ye, L.; Hu, L.; Jeong, S.; Elias, P.M.; Lv, C. Improvements in epidermal function prevent relapse of psoriasis: A self-controlled study. Clin. Exp. Dermatol. 2019, 44, 654–657. [Google Scholar] [CrossRef]
- Osborne, D.W.; Ward, A.J.; O’Neill, K.J. Microemulsions as topical drug delivery vehicles: In-vitro transdermal studies of a model hydrophilic drug. J. Pharm. Pharmacol. 1991, 43, 450–454. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef]
- Niu, Z.; Conejos-Sanchez, I.; Griffin, B.T.; O’Driscoll, C.M.; Alonso, M.J. Lipid-based nanocarriers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016, 106, 337–354. [Google Scholar] [CrossRef]
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Subongkot, T.; Ngawhirunpat, T. Development of a novel microemulsion for oral absorption enhancement of all-trans retinoic acid. Int. J. Nanomed. 2017, 12, 5585–5599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naoui, W.; Bolzinger, M.A.; Fenet, B.; Pelletier, J.; Valour, J.P.; Kalfat, R.; Chevalier, Y. Microemulsion microstructure influences the skin delivery of an hydrophilic drug. Pharm. Res. 2011, 28, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Michniak-Kohn, B.B. Investigation of microemulsion and microemulsion gel formulations for dermal delivery of clotrimazole. Int. J. Pharm. 2018, 536, 345–352. [Google Scholar] [CrossRef]
- Purdon, C.H.; Azzi, C.G.; Zhang, J.; Smith, E.W.; Maibach, H.I. Penetration enhancement of transdermal delivery--current permutations and limitations. Crit. Rev. Ther. Drug Carrier Syst. 2004, 21, 97–132. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Benfeldt, E.; Holmgaard, R. Penetration through the Skin Barrier. Curr. Probl. Dermatol. 2016, 49, 103–111. [Google Scholar] [CrossRef]
- Berardesca, E.; Vignoli, G.P.; Farinelli, N.; Vignini, M.; Distante, F.; Rabbiosi, G. Non-invasive evaluation of topical calcipotriol versus clobetasol in the treatment of psoriasis. Acta Derm. Venereol. 1994, 74, 302–304. [Google Scholar] [CrossRef]
- Berardesca, E.; Maibach, H.I. Transepidermal water loss and skin surface hydration in the non invasive assessment of stratum corneum function. Derm. Beruf. Umwelt. 1990, 38, 50–53. [Google Scholar]
- Rim, J.H.; Jo, S.J.; Park, J.Y.; Park, B.D.; Youn, J.I. Electrical measurement of moisturizing effect on skin hydration and barrier function in psoriasis patients. Clin. Exp. Dermatol. 2005, 30, 409–413. [Google Scholar] [CrossRef]
- Tagami, H.; Kobayashi, H.; Kikuchi, K. A portable device using a closed chamber system for measuring transepidermal water loss: Comparison with the conventional method. Skin Res. Technol. 2002, 8, 7–12. [Google Scholar]
- Nikam, V.N.; Monteiro, R.C.; Dandakeri, S.; Bhat, R.M. Transepidermal Water Loss in Psoriasis: A Case-control Study. Indian Dermatol. Online J. 2019, 10, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Koga, Y.; Kainoh, M. Anti-IL-12/IL-23p40 antibody ameliorates dermatitis and skin barrier dysfunction in mice with imiquimod-induced psoriasis-like dermatitis. Eur. J. Pharmacol. 2018, 828, 26–30. [Google Scholar] [CrossRef] [PubMed]





| Properties (Unit) | Formulation A |
|---|---|
| Composition | O:S:W = 1:6:3 |
| Droplet size (nm) | 696.2 ± 188.3 |
| Size distribution (PI) | 0.435 ± 0.004 |
| Zeta potential (mV) | −14.95 ± 0.64 |
| Viscosity (cP) | 3112.3 ± 5.8 |
| Electronic conductivity (μS/cm) | 24.15 ± 0.07 |
| Parameters (Unit) | Formulation A | EtOH |
|---|---|---|
| Js (ng/cm2 h) | 574.44 ± 280.00 * | 52.66 ± 8.09 |
| tlag (hr) | 1.78 ± 0.32 | 0.82 ± 0.14 * |
| Q (ng/cm2) | 2411.23 ± 877.94 * | 305.14 ± 65.05 |
| Kp (×10−3 cm/h) | 1.91 ± 0.93 * | 0.18 ± 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.-W.; Cheng, Y.-P.; Liu, C.-Y.; Thong, H.-Y.; Huang, C.-J.; Lo, Y.; Wu, C.-Y.; Jee, S.-H. Salvianolic Acid B in Microemulsion Formulation Provided Sufficient Hydration for Dry Skin and Ameliorated the Severity of Imiquimod-induced Psoriasis-like Dermatitis in Mice. Pharmaceutics 2020, 12, 457. https://doi.org/10.3390/pharmaceutics12050457
Guo J-W, Cheng Y-P, Liu C-Y, Thong H-Y, Huang C-J, Lo Y, Wu C-Y, Jee S-H. Salvianolic Acid B in Microemulsion Formulation Provided Sufficient Hydration for Dry Skin and Ameliorated the Severity of Imiquimod-induced Psoriasis-like Dermatitis in Mice. Pharmaceutics. 2020; 12(5):457. https://doi.org/10.3390/pharmaceutics12050457
Chicago/Turabian StyleGuo, Jiun-Wen, Yu-Pin Cheng, Chih-Yi Liu, Haw-Yueh Thong, Chi-Jung Huang, Yang Lo, Chen-Yu Wu, and Shiou-Hwa Jee. 2020. "Salvianolic Acid B in Microemulsion Formulation Provided Sufficient Hydration for Dry Skin and Ameliorated the Severity of Imiquimod-induced Psoriasis-like Dermatitis in Mice" Pharmaceutics 12, no. 5: 457. https://doi.org/10.3390/pharmaceutics12050457
APA StyleGuo, J. -W., Cheng, Y. -P., Liu, C. -Y., Thong, H. -Y., Huang, C. -J., Lo, Y., Wu, C. -Y., & Jee, S. -H. (2020). Salvianolic Acid B in Microemulsion Formulation Provided Sufficient Hydration for Dry Skin and Ameliorated the Severity of Imiquimod-induced Psoriasis-like Dermatitis in Mice. Pharmaceutics, 12(5), 457. https://doi.org/10.3390/pharmaceutics12050457