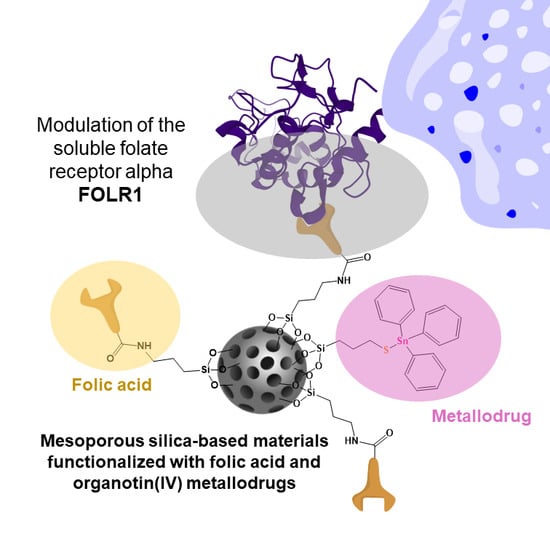
Role of Folic Acid in the Therapeutic Action of Nanostructured Porous Silica Functionalized with Organotin(IV) Compounds against Different Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Remarks on the Synthesis and Characterization of the Materials
2.2. Synthesis of Mesoporous Silica Nanoparticles (MSNs)
2.3. Synthesis of Porous Micronic Silica Particles of the MSU-2 Type (MSU-2)
2.4. Functionalization of Silica Materials with Amino Ligand—Synthesis of MSN-AP or MSU-2-AP
2.5. Incorporation of Folate Fragment—Synthesis of MSN-AP-FA or MSU-2-AP-FA
2.6. Incorporation of a High Quantity of Folate Fragment—Synthesis of MSN-AP-FA25 or MSU-2-AP-FA25
2.7. Incorporation of the Cytotoxic Fragment SnPh3—Synthesis of MSN-Sn or MSU-2-Sn
2.8. Incorporation of the Cytotoxic Fragment SnPh3—Synthesis of MSN-AP-FA-Sn, MSN-AP-FA25-Sn, MSU-2-AP-FA-Sn and MSU-2-AP-FA25-Sn
2.9. Cell Growth Inhibition
2.10. Modulation of Soluble Folate Receptor Alpha
3. Results and Discussion
3.1. Synthesis and Characterization of Starting Porous Silica Materials
3.2. Synthesis and Characterization of Functionalized Materials
3.3. Anticancer Potential of the Materials Against Different Cancer Cell Lines
3.4. Modulation of the Soluble Folate Receptor Alpha (FOLR1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Worldwide Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer (accessed on 4 April 2020).
- Cancer Tomorrow. Available online: http://gco.iarc.fr/tomorrow/home (accessed on 4 April 2020).
- Jackson, S.E.; Chester, J.D. Personalised cancer medicine. Int. J. Cancer 2015, 137, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Dumbrava, E.I.; Meric-Bernstam, F. Personalized cancer therapy—Leveraging a knowledge base for clinical decision-making. Cold Spring Harb. Mol. Case Stud. 2018, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.H.W.; Kuo, M.T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget 2017, 8, 62742–62758. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, C.; Friedman, L.S.; Hager, J.H. Hormone-Targeted Therapy and Resistance. Annu. Rev. Cancer Biol. 2018, 2, 291–312. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef]
- Rosenberg, B.; VanCamp, L. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Res. 1970, 30, 1799–1802. [Google Scholar]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug. Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [Green Version]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Wani, W.A.; Prashar, S.; Shreaz, S.; Gómez-Ruiz, S. Nanostructured materials functionalized with metal complexes: In search of alternatives for administering anticancer metallodrugs. Coord. Chem. Rev. 2016, 312, 67–98. [Google Scholar] [CrossRef]
- Ellahioui, Y.; Prashar, S.; Gómez-Ruiz, S. Anticancer Applications and Recent Investigations of Metallodrugs Based on Gallium, Tin and Titanium. Inorganics 2017, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Poursharifi, M.; Wlodarczyk, M.T.; Mieszawska, A.J. Nano-Based Systems and Biomacromolecules as Carriers for Metallodrugs in Anticancer Therapy. Inorganics 2019, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Ellahioui, Y.; Prashar, S.; Gómez-Ruiz, S. A Short Overview on the Biomedical Applications of Silica, Alumina and Calcium Phosphate-based Nanostructured Materials. Curr. Med. Chem. 2016, 23, 4450–4467. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; Gómez-Ruiz, S.; Žižak, Ž.; Sierra, I.; Prashar, S.; del Hierro, I.; Fajardo, M.; Juranić, Z.D.; Kaluđerović, G.N. A New Generation of Anticancer Drugs: Mesoporous Materials Modified with Titanocene Complexes. Chem. Eur. J. 2009, 15, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Kaluderović, G.N.; Pérez-Quintanilla, D.; Zizak, Z.; Juranić, Z.D.; Gómez-Ruiz, S. Improvement of cytotoxicity of titanocene-functionalized mesoporous materials by the increase of the titanium content. Dalton Trans. 2010, 39, 2597–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaluđerović, G.N.; Pérez-Quintanilla, D.; Sierra, I.; Prashar, S.; del Hierro, I.; Žižak, Ž.; Juranić, Z.D.; Fajardo, M.; Gómez-Ruiz, S. Study of the influence of the metal complex on the cytotoxic activity of titanocene-functionalized mesoporous materials. J. Mater. Chem. 2010, 20, 806–814. [Google Scholar] [CrossRef]
- García-Peñas, A.; Gómez-Ruiz, S.; Pérez-Quintanilla, D.; Paschke, R.; Sierra, I.; Prashar, S.; del Hierro, I.; Kaluđerović, G.N. Study of the cytotoxicity and particle action in human cancer cells of titanocene-functionalized materials with potential application against tumors. J. Inorg. Biochem. 2012, 106, 100–110. [Google Scholar] [CrossRef]
- Bulatović, M.Z.; Maksimović-Ivanić, D.; Bensing, C.; Gómez-Ruiz, S.; Steinborn, D.; Schmidt, H.; Mojić, M.; Korać, A.; Golić, I.; Pérez-Quintanilla, D.; et al. Organotin(IV)-loaded mesoporous silica as a biocompatible strategy in cancer treatment. Angew. Chem. Int. Ed. Engl. 2014, 53, 5982–5987. [Google Scholar] [CrossRef]
- Ceballos-Torres, J.; Virag, P.; Cenariu, M.; Prashar, S.; Fajardo, M.; Fischer-Fodor, E.; Gómez-Ruiz, S. Anti-cancer Applications of Titanocene-Functionalised Nanostructured Systems: An Insight into Cell Death Mechanisms. Chem. Eur. J. 2014, 20, 10811–10828. [Google Scholar] [CrossRef]
- Ceballos-Torres, J.; Prashar, S.; Fajardo, M.; Chicca, A.; Gertsch, J.; Pinar, A.B.; Gómez-Ruiz, S. Ether-Substituted Group 4 Metallocene Complexes: Cytostatic Effects and Applications in Ethylene Polymerization. Organometallics 2015, 34, 2522–2532. [Google Scholar] [CrossRef]
- Bensing, C.; Mojić, M.; Gómez-Ruiz, S.; Carralero, S.; Dojčinović, B.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Evaluation of functionalized mesoporous silica SBA-15 as a carrier system for Ph3Sn(CH2)3OH against the A2780 ovarian carcinoma cell line. Dalton Trans. 2016, 45, 18984–18993. [Google Scholar] [CrossRef]
- Díaz-García, D.; Cenariu, D.; Pérez, Y.; Cruz, P.; del Hierro, I.; Prashar, S.; Fischer-Fodor, E.; Gómez-Ruiz, S. Modulation of the mechanism of apoptosis in cancer cell lines by treatment with silica-based nanostructured materials functionalized with different metallodrugs. Dalton Trans. 2018, 47, 12284–12299. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; García-Peñas, A.; Prashar, S.; Rodríguez-Diéguez, A.; Fischer-Fodor, E. Anticancer Applications of Nanostructured Silica-Based Materials Functionalized with Titanocene Derivatives: Induction of Cell Death Mechanism through TNFR1 Modulation. Materials 2018, 11, 224. [Google Scholar] [CrossRef] [Green Version]
- Del Hierro, I.; Gómez-Ruiz, S.; Pérez, Y.; Cruz, P.; Prashar, S.; Fajardo, M. Mesoporous SBA-15 modified with titanocene complexes and ionic liquids: Interactions with DNA and other molecules of biological interest studied by solid state electrochemical techniques. Dalton Trans. 2018, 47, 12914–12932. [Google Scholar] [CrossRef]
- Díaz-García, D.; Ardiles, P.R.; Prashar, S.; Rodríguez-Diéguez, A.; Páez, P.L.; Gómez-Ruiz, S. Preparation and Study of the Antibacterial Applications and Oxidative Stress Induction of Copper Maleamate-Functionalized Mesoporous Silica Nanoparticles. Pharmaceutics 2019, 11, 30. [Google Scholar] [CrossRef] [Green Version]
- Ellahioui, Y.; Patra, M.; Mari, C.; Kaabi, R.; Karges, J.; Gasser, G.; Gómez-Ruiz, S. Mesoporous silica nanoparticles functionalised with a photoactive ruthenium(ii) complex: Exploring the formulation of a metal-based photodynamic therapy photosensitiser. Dalton Trans. 2019, 48, 5940–5951. [Google Scholar] [CrossRef]
- Edeler, D.; Kaluđerović, M.R.; Dojčinović, B.; Schmidt, H.; Kaluđerović, G.N. SBA-15 mesoporous silica particles loaded with cisplatin induce senescence in B16F10 cells. RSC Adv. 2016, 6, 111031–111040. [Google Scholar] [CrossRef] [Green Version]
- Rojas, S.; Carmona, F.J.; Barea, E.; Maldonado, C.R. Inorganic mesoporous silicas as vehicles of two novel anthracene-based ruthenium metalloarenes. J. Inorg. Biochem. 2017, 166, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Edeler, D.; Arlt, S.; Petković, V.; Ludwig, G.; Drača, D.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Delivery of [Ru(η6-p-cymene)Cl2{Ph2P(CH2)3SPh-κP}] using unfunctionalized and mercapto functionalized SBA-15 mesoporous silica: Preparation, characterization and in vitro study. J. Inorg. Biochem. 2018, 180, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Maksimović-Ivanić, D.; Bulatović, M.; Edeler, D.; Bensing, C.; Golić, I.; Korać, A.; Kaluđerović, G.N.; Mijatović, S. The interaction between SBA-15 derivative loaded with Ph3Sn(CH2)6OH and human melanoma A375 cell line: Uptake and stem phenotype loss. J. Biol. Inorg. Chem. 2019, 24, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Edeler, D.; Drača, D.; Petković, V.; Natalio, F.; Maksimović-Ivanić, D.; Mijatović, S.; Schmidt, H.; Kaluđerović, G.N. Impact of the mesoporous silica SBA-15 functionalization on the mode of action of Ph3Sn(CH2)6OH. Mater. Sci. Eng. C 2019, 100, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, D.; Sommerova, L.; Martisova, A.; Skoupilova, H.; Prashar, S.; Vaculovic, T.; Kanicky, V.; del Hierro, I.; Hrstka, R.; Gómez-Ruiz, S. Mesoporous silica nanoparticles functionalized with a dialkoxide diorganotin(IV) compound: In search of more selective systems against cancer cells. Microporous Mesoporous Mater. 2020, 300, 110154. [Google Scholar] [CrossRef]
- Ovejero Paredes, K.; Díaz-García, D.; García-Almodóvar, V.; Lozano Chamizo, L.; Marciello, M.; Díaz-Sánchez, M.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer. Cancers 2020, 12, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Fang, G.; Wang, X.; Zeng, F.; Xiang, Y.; Wu, S. Targeted anticancer prodrug with mesoporous silica nanoparticles as vehicles. Nanotechnology 2011, 22, 455102. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Z.; Zink, J.I.; Tamanoi, F. In vivo tumor suppression efficacy of mesoporous silica nanoparticles-based drug-delivery system: Enhanced efficacy by folate modification. Nanomedicine 2012, 8, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Khosravian, P.; Shafiee Ardestani, M.; Khoobi, M.; Ostad, S.N.; Dorkoosh, F.A.; Akbari Javar, H.; Amanlou, M. Mesoporous silica nanoparticles functionalized with folic acid/methionine for active targeted delivery of docetaxel. Onco Targets Ther. 2016, 9, 7315–7330. [Google Scholar] [CrossRef] [Green Version]
- Knežević, N.Ž.; Mrđanović, J.; Borišev, I.; Milenković, S.; Janaćković, Đ.; Cunin, F.; Djordjevic, A. Hydroxylated fullerene-capped, vinblastine-loaded folic acid-functionalized mesoporous silica nanoparticles for targeted anticancer therapy. RSC Adv. 2016, 6, 7061–7065. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Liu, Q.; Yang, L.; Zhu, R.; Yu, C.; Wang, S. Rational Design of Multifunctional Dendritic Mesoporous Silica Nanoparticles to Load Curcumin and Enhance Efficacy for Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 26511–26523. [Google Scholar] [CrossRef]
- Khattabi, A.M.; Talib, W.H.; Alqdeimat, D.A. A targeted drug delivery system of anti-cancer agents based on folic acid-cyclodextrin-long polymer functionalized silica nanoparticles. J. Drug Deliv. Sci. Technol. 2017, 41, 367–374. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, N.; Liu, L.; Li, J.; Zhang, Y.; Shen, C.; Shezad, K.; Zhang, L.; Zhu, J.; Tao, J. Dacarbazine-Loaded Hollow Mesoporous Silica Nanoparticles Grafted with Folic Acid for Enhancing Antimetastatic Melanoma Response. ACS Appl. Mater. Interfaces 2017, 9, 21673–21687. [Google Scholar] [CrossRef]
- Qu, W.; Meng, B.; Yu, Y.; Wang, S. Folic acid-conjugated mesoporous silica nanoparticles for enhanced therapeutic efficacy of topotecan in retina cancers. Int. J. Nanomed. 2018, 13, 4379–4389. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Li, Y.; Xiao, Y.; Zhao, Z.; Zhang, C.; Wang, J.; Li, H.; Liu, F.; He, N.; Yuan, Y.; et al. Folate-conjugated nanobubbles selectively target and kill cancer cells via ultrasound-triggered intracellular explosion. Biomaterials 2018, 181, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zong, Y.; Yang, Z.; Luo, M.; Li, G.; Yingsa, W.; Cao, Y.; Xiao, M.; Kong, T.; He, J.; et al. Dual-Targeted Controlled Delivery Based on Folic Acid Modified Pectin-Based Nanoparticles for Combination Therapy of Liver Cancer. ACS Sustain. Chem. Eng. 2019, 7, 3614–3623. [Google Scholar] [CrossRef]
- Poltavets, Y.I.; Zhirnik, A.S.; Zavarzina, V.V.; Semochkina, Y.P.; Shuvatova, V.G.; Krasheninnikova, A.A.; Aleshin, S.V.; Dronov, D.O.; Vorontsov, E.A.; Balabanyan, V.Y.; et al. In vitro anticancer activity of folate-modified docetaxel-loaded PLGA nanoparticles against drug-sensitive and multidrug-resistant cancer cells. Cancer Nanotechnol. 2019, 10. [Google Scholar] [CrossRef]
- De Oliveira Silva, J.; Fernandes, R.S.; Ramos Oda, C.M.; Ferreira, T.H.; Machado Botelho, A.F.; Martins Melo, M.; de Miranda, M.C.; Assis Gomes, D.; Dantas Cassali, G.; Townsend, D.M.; et al. Folate-coated, long-circulating and pH-sensitive liposomes enhance doxorubicin antitumor effect in a breast cancer animal model. Biomed. Pharmacother. 2019, 118, 109323. [Google Scholar] [CrossRef] [PubMed]
- Iturrioz-Rodríguez, N.; Correa-Duarte, M.A.; Fanarraga, M.L. Controlled drug delivery systems for cancer based on mesoporous silica nanoparticles. Int. J. Nanomed. 2019, 14, 3389–3401. [Google Scholar] [CrossRef] [Green Version]
- Malekmohammadi, S.; Hadadzadeh, H.; Amirghofran, Z. Preparation of folic acid-conjugated dendritic mesoporous silica nanoparticles for pH-controlled release and targeted delivery of a cyclometallated gold(III) complex as an antitumor agent. J. Mol. Liq. 2018, 265, 797–806. [Google Scholar] [CrossRef]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, L.C.; Keeney, G.L.; Lingle, W.L.; Christianson, T.J.H.; Varghese, B.; Hillman, D.; Oberg, A.L.; Low, P.S. Folate receptor overexpression is associated with poor outcome in breast cancer. Int. J. Cancer 2007, 121, 938–942. [Google Scholar] [CrossRef]
- Wibowo, A.S.; Singh, M.; Reeder, K.M.; Carter, J.J.; Kovach, A.R.; Meng, W.; Ratnam, M.; Zhang, F.; Dann, C.E. Structures of human folate receptors reveal biological trafficking states and diversity in folate and antifolate recognition. Proc. Natl. Acad. Sci. USA 2013, 110, 15180–15188. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.-S. Folate Receptor-Targeted Diagnostics and Therapeutics for Inflammatory Diseases. Immune Netw. 2016, 16, 337–343. [Google Scholar] [CrossRef]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.F.; Greibe, E.; Skovbjerg, S.; Rohde, S.; Kristensen, A.C.M.; Jensen, T.R.; Stentoft, C.; Kjær, K.H.; Kronborg, C.S.; Martensen, P.M. Folic acid mediates activation of the pro-oncogene STAT3 via the Folate Receptor alpha. Cell Signal. 2015, 27, 1356–1368. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, L.E.; Brenton, J.D.; Parkinson, C.; Whitaker, H.C.; Piskorz, A.M.; Csizmadi, I.; Robson, P.J. Conditions Associated with Circulating Tumor-Associated Folate Receptor 1 Protein in Healthy Men and Women. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Basal, E.; Eghbali-Fatourechi, G.Z.; Kalli, K.R.; Hartmann, L.C.; Goodman, K.M.; Goode, E.L.; Kamen, B.A.; Low, P.S.; Knutson, K.L. Functional folate receptor alpha is elevated in the blood of ovarian cancer patients. PLoS ONE 2009, 4, e6292. [Google Scholar] [CrossRef] [PubMed]
- Leung, F.; Dimitromanolakis, A.; Kobayashi, H.; Diamandis, E.P.; Kulasingam, V. Folate-receptor 1 (FOLR1) protein is elevated in the serum of ovarian cancer patients. Clin. Biochem. 2013, 46, 1462–1468. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Erba, E.; D’Incalci, M.; Bottero, F.; Canevari, S.; Tomassetti, A. Role of membrane folate-binding protein in the cytotoxicity of 5,10-dideazatetrahydrofolic acid in human ovarian carcinoma cell lines in vitro. Br. J. Cancer 1996, 73, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Peng, M.; Li, N.; Song, Q.; Yao, Y.; Xu, B.; Liu, H.; Ruan, P. Combined use of EpCAM and FRα enables the high-efficiency capture of circulating tumor cells in non-small cell lung cancer. Sci. Rep. 2018, 8, 1188. [Google Scholar] [CrossRef] [Green Version]
- Tsukihara, H.; Tsunekuni, K.; Takechi, T. Folic Acid-Metabolizing Enzymes Regulate the Antitumor Effect of 5-Fluoro-2′-Deoxyuridine in Colorectal Cancer Cell Lines. PLoS ONE 2016, 11, e0163961. [Google Scholar] [CrossRef] [PubMed]
- Theti, D.S.; Jackman, A.L. The role of alpha-folate receptor-mediated transport in the antitumor activity of antifolate drugs. Clin. Cancer Res. 2004, 10, 1080–1089. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Quintanilla, D.; Sánchez, A.; del Hierro, I.; Fajardo, M.; Sierra, I. Synthesis and characterization of novel mesoporous silicas of the MSU-X family for environmental applications. J. Nanosci. Nanotechnol. 2009, 9, 4901–4909. [Google Scholar] [CrossRef] [PubMed]
- Bollu, V.S.; Barui, A.K.; Mondal, S.K.; Prashar, S.; Fajardo, M.; Briones, D.; Rodríguez-Diéguez, A.; Patra, C.R.; Gómez-Ruiz, S. Curcumin-loaded silica-based mesoporous materials: Synthesis, characterization and cytotoxic properties against cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 393–410. [Google Scholar] [CrossRef]
- Kotcherlakota, R.; Barui, A.K.; Prashar, S.; Fajardo, M.; Briones, D.; Rodríguez-Diéguez, A.; Patra, C.R.; Gómez-Ruiz, S. Curcumin loaded mesoporous silica: An effective drug delivery system for cancer treatment. Biomater. Sci. 2016, 4, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Fernández, B.; Oyarzabal, I.; Fischer-Fodor, E.; Macavei, S.; Sánchez, I.; Seco, J.M.; Gómez-Ruiz, S.; Rodríguez-Diéguez, A. Multifunctional applications of a dysprosium-based metal–organic chain with single-ion magnet behaviour. CrystEngComm 2016, 18, 8718–8721. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Dhanasekaran, S. Augmented cytotoxic effects of paclitaxel by curcumin induced overexpression of folate receptor-α for enhanced targeted drug delivery in HeLa cells. Phytomedicine 2019, 56, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.R.; Piroyan, A.; Ganta, S.; Morse, A.B.; Candiloro, K.M.; Solon, A.L.; Nack, A.H.; Galati, C.A.; Bora, C.; Maglaty, M.A.; et al. In Vitro and In Vivo evaluation of a novel folate-targeted theranostic nanoemulsion of docetaxel for imaging and improved anticancer activity against ovarian cancers. Cancer Biol. Ther. 2018, 19, 554–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Material | SBET (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| MSN | 1380 | 1.17 | 3.39 |
| MSU-2 | 848 | 0.72 | 3.41 |
| Material | AP (TG) mmol/g | FA (TG) mmol/g | %Sn ± SD (XRF) |
|---|---|---|---|
| MSN-AP-FA-Sn | 1.31 | 0.51 | 7.56 ± 0.03 |
| MSU-2-AP-FA-Sn | 0.98 | 0.17 | 6.12 ± 0.03 |
| MSN-AP-FA25-Sn | n.a.a | n.a. | 5.68 ± 0.03 |
| MSU-2-AP-FA25-Sn | n.a. | n.a. | 5.91 ± 0.04 |
| MSN-Sn | - | - | 13.90 ± 0.03 |
| MSU-2-Sn | - | - | 14.10 ± 0.03 |
| Material/Cell Line | OVCAR-3 | DLD-1 | A2780 | A431 | HaCaT |
|---|---|---|---|---|---|
| MSN | 267.11 ± 15.14 | >500 | >500 | >500 | 440.45 ±17.73 |
| MSN-AP | 223.09 ± 18.55 | >500 | >500 | >500 | 358.40 ± 41.88 |
| MSN-AP-FA | 177.78 ± 11.79 | 392.07 ± 24.10 | >500 | 429.55 ± 34.03 | 360.81 ± 32.62 |
| MSN-AP-FA25 | 182.65 ± 20.32 | 327.53 ± 17.05 | 341.91 ± 23.31 | 432.76 ± 28.02 | 342.65 ± 20.05 |
| MSN-AP-FA-Sn | 50.36 ± 3.82 | 82.32 ± 9.44 | 155.19 ± 6.59 | 102.5 ± 7.71 | 134.18 ± 15.91 |
| MSN-AP-FA25-Sn | 22.49 ± 1.43 | 67.70 ± 4.08 | 143.63 ± 9.77 | 94.01 ± 5.62 | 152.80 ± 9.88 |
| MSN-Sn | 101.55 ± 2.11 | 184.03 ± 25.64 | 198.38 ± 9.99 | 87.81 ± 13.47 | 123.98 ± 20.41 |
| MSU-2 | 183.71 ± 26.85 | 266.43 ± 10.27 | 348.46 ± 29.72 | 112.40 ± 20.13 | 301.92 ± 16.12 |
| MSU-2-AP | 164.80 ± 15.00 | 235.55 ± 9.04 | 329.25 ± 37.33 | 74.26 ± 7.03 | 256.72 ± 30.52 |
| MSU-2-AP-FA | 96.75 ± 8.31 | 189.25 ± 26.85 | 296.54 ± 20.54 | 90.18 ± 13.86 | 233.20 ± 16.90 |
| MSU-2-AP-FA25 | 91.14 ± 10.64 | 103.23 ± 8.00 | 288.62 ± 12.27 | 88.33 ± 4.6 | 239.48 ± 17.82 |
| MSU-2-AP-FA-Sn | 21.02 ± 4.16 | 49.60 ± 8.3 | 146.05 ± 9.08 | 53.78 ± 11.25 | 144.48 ± 6.81 |
| MSU-2-AP-FA25-Sn | 9.35 ± 1.13 | 20.64 ± 0.88 | 96.83 ± 6.42 | 65.30 ± 5.80 | 115.10 ± 9.72 |
| MSU-2-Sn | 79.61 ± 7.55 | 107.04 ± 11.45 | 181.98 ± 10.69 | 59.19 ± 18.97 | 124.32 ± 10.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-García, D.; Montalbán-Hernández, K.; Mena-Palomo, I.; Achimas-Cadariu, P.; Rodríguez-Diéguez, A.; López-Collazo, E.; Prashar, S.; Ovejero Paredes, K.; Filice, M.; Fischer-Fodor, E.; et al. Role of Folic Acid in the Therapeutic Action of Nanostructured Porous Silica Functionalized with Organotin(IV) Compounds against Different Cancer Cell Lines. Pharmaceutics 2020, 12, 512. https://doi.org/10.3390/pharmaceutics12060512
Díaz-García D, Montalbán-Hernández K, Mena-Palomo I, Achimas-Cadariu P, Rodríguez-Diéguez A, López-Collazo E, Prashar S, Ovejero Paredes K, Filice M, Fischer-Fodor E, et al. Role of Folic Acid in the Therapeutic Action of Nanostructured Porous Silica Functionalized with Organotin(IV) Compounds against Different Cancer Cell Lines. Pharmaceutics. 2020; 12(6):512. https://doi.org/10.3390/pharmaceutics12060512
Chicago/Turabian StyleDíaz-García, Diana, Karla Montalbán-Hernández, Irene Mena-Palomo, Patriciu Achimas-Cadariu, Antonio Rodríguez-Diéguez, Eduardo López-Collazo, Sanjiv Prashar, Karina Ovejero Paredes, Marco Filice, Eva Fischer-Fodor, and et al. 2020. "Role of Folic Acid in the Therapeutic Action of Nanostructured Porous Silica Functionalized with Organotin(IV) Compounds against Different Cancer Cell Lines" Pharmaceutics 12, no. 6: 512. https://doi.org/10.3390/pharmaceutics12060512
APA StyleDíaz-García, D., Montalbán-Hernández, K., Mena-Palomo, I., Achimas-Cadariu, P., Rodríguez-Diéguez, A., López-Collazo, E., Prashar, S., Ovejero Paredes, K., Filice, M., Fischer-Fodor, E., & Gómez-Ruiz, S. (2020). Role of Folic Acid in the Therapeutic Action of Nanostructured Porous Silica Functionalized with Organotin(IV) Compounds against Different Cancer Cell Lines. Pharmaceutics, 12(6), 512. https://doi.org/10.3390/pharmaceutics12060512